Meningitis is a serious infectious disease that, in the absence of timely medical care, can lead to the death of the patient. The disease mainly develops in people with weakened immune systems: children, elderly people, HIV-infected people, cancer patients. Therefore, many parents are interested in whether it is possible to protect their child from this disease. At the moment, vaccination against meningitis is the only reliable method of preventing infection. It is worth considering in more detail the features of vaccination and how necessary it is.
Why is meningitis dangerous?
Meningitis is an inflammation of the membranes of the brain or spinal cord of infectious origin. The disease is characterized by rapid development - in the absence of medical care, the patient may lose vision and hearing within 24 hours. The cause of meningitis is the introduction of Haemophilus influenzae, meningococcus, and pneumococcus into the brain with blood. In newborns, the causative agent of infection can also be Escherichia coli, Klebsiella, and Enterococcus.
Important! Meningitis in 60% of cases is of a viral nature; in such cases, the infectious process is provoked by the Coxsackie or ECHO viruses.
Source of the disease
The source of infection is sick people who have clinically pronounced signs of the disease, and carriers of the pathogen. How to identify infected patients and carriers of meningococcal infection? People who secrete meningococcus can usually be detected during a mass examination of the source of infection, during the taking of smears from the nasopharyngeal mucosa as part of medical examinations. It is impossible to clinically determine the carrier of meningococcal infection, because the person does not have any signs of the disease.
Important! The risk of contracting meningitis in patients who have had meningitis or been vaccinated is 0.1%.
Symptoms and danger of the disease
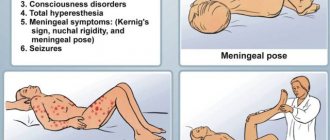
The first symptoms of meningitis are similar to the common cold, which complicates diagnosis. An infectious disease leads to an increase in body temperature, the development of a severe headache, vomiting and nausea. In some cases, a hemorrhagic rash appears on the skin.
The danger of meningitis lies in the possibility of developing cerebral edema and secondary encephalitis (infection of brain tissue). As a result, meningoencephalitis develops, which is characterized by severe neurological symptoms. It persists for a long time after recovery and often becomes a cause of disability for the patient.
However, the greatest danger is posed by a brain abscess that occurs as a consequence of secondary bacterial meningitis against the background of pathologies of the ENT organs (sinusitis, otitis media, sinusitis). The pathology provokes the development of cerebral edema and displacement of the midline structures. Therefore, patients need not only drug treatment, but also surgical intervention.
Reaction to vaccination against meningitis in children
In children, reactions to the meningitis vaccine may include:
- fever, chills, increased body temperature not higher than 37.50C;
- drowsiness;
- feeling of muscle soreness;
- local reactions in the form of swelling in the injection area, redness or mild rash.
Adverse reactions should subside within two days; the lump in the injection area completely disappears after 14 days. If symptoms do not disappear, you should immediately seek help from a doctor.
You can get advice and make an appointment with a neurologist by calling the Yusupov Hospital.
Who needs immunization?
The infectious disease is more common in childhood, which is associated with an imperfect immune system. Meningitis is diagnosed in adults against the background of immunodeficiency states: elderly people, HIV-infected people, during chemotherapy. Therefore, vaccination against meningitis is indicated for the following groups of patients:
- Children who were born prematurely;
- Children and adults who have frequent seasonal respiratory infections;
- Children under 2 years old;
- Families with more than 1 child;
- Children who have been bottle-fed or mixed-fed since birth;
- Patients with advanced dental pathologies;
- A history of recurrent bronchitis, pneumonia, otitis, sinusitis;
- Medical and laboratory staff;
- Children who regularly attend children's groups (kindergarten, early development groups, dances);
- Patients with severe immunodeficiency conditions (HIV-infected, cancer patients);
- Conscripts and students who will live in the dormitory;
- People with severe cardiovascular pathologies;
- Travelers and tourists who go to regions with a high probability of infection;
- Patients who have had their spleen removed or have anatomical defects of the skull;
- People who have been in contact with infected patients or carriers of meningococcal infection, Haemophilus influenzae.
The meningitis vaccine is also recommended for women who are planning a pregnancy.
Important! Vaccination against meningitis in children helps reduce the risk of developing acute respiratory infections. Therefore, vaccination is indicated for children who are often ill.
In many developed countries, vaccination against meningitis has become mandatory, which has made it possible to almost completely overcome the infection. Vaccination against meningitis is not included in the National Vaccination Calendar in Russia due to the high cost of vaccine preparations. Therefore, free immunization of the population is carried out only in the following cases:
- The development of an epidemic when the incidence rate exceeds 20 patients per 100 thousand people;
- If a child is found in the group who is suspected of having meningitis. In this case, vaccination against meningitis is necessary for children in contact with it;
- The patient lives in a region with a high incidence of disease;
- A child with severe immunodeficiency.
In other cases, parents and patients must independently purchase vaccine preparations from the pharmacy chain.
SPECIAL INSTRUCTIONS
Menactra vaccine should not be administered intravenously, subcutaneously, or intradermally because there is no data on the safety and effectiveness of the vaccine when administered subcutaneously, intravenously, or intradermally.
Do not mix Menactra vaccine in the same syringe with other vaccines or medications.
The vaccine has not been studied in persons with thrombocytopenia or bleeding disorders. As with other vaccines administered intramuscularly, the benefit versus risk of using the vaccine should be assessed in individuals at increased risk of bleeding from intramuscular injection.
The risk of developing Guillain-Barré syndrome (GBS) after vaccination with Menactra was assessed in a post-marketing retrospective cohort study. Cases of the development of GBS have been described, characterized by a temporal connection with the administration of the Menactra vaccine. Individuals who have previously been diagnosed with GBS may be at increased risk of developing this condition after receiving the Menactra vaccine. The decision to use Menactra vaccine in this situation should be made after assessing the potential benefits and risks.
The vaccine is not intended for the prevention of meningitis caused by other microorganisms or for the prevention of invasive meningococcal infection caused by meningococcal serogroup B in persons with an impaired immune status, as well as against the background of immunosuppressive therapy, a reduced immune response to the administration of the Menactra vaccine may be observed.
As with any vaccination, protective immunity may not develop in all 100 vaccinated people.
Before administering a vaccine, the health care provider or prescriber should inform the patient, parent, guardian, or other responsible adult of the potential benefits and risks associated with receiving the vaccine.
INFLUENCE ON THE ABILITY TO DRIVE VEHICLES AND OPERATE MACHINES
No studies have been conducted to study the effect of the vaccine on the ability to drive a car or use other machinery.
Features of vaccination
The following features of immunoprophylaxis are distinguished:
- Haemophilus influenzae infection is characterized by a severe course, complications often develop. Mostly children aged 5-6 years old suffer from Haemophilus influenzae. The effectiveness of the vaccine reaches 95%, revaccination leads to an exponential increase in the number of antibodies;
- Pneumococci cause meningitis in children under 2 years of age and patients over 65 years of age. The disease is often combined with pneumonia. Mass immunoprophylaxis can reduce the risk of developing infection by 80%;
- The development of meningococcal infection is observed mainly in infants under 1 year of age. The causative agent is meningococci types A, B, C, W-135, Y. Vaccination against meningococcal infection helps to form an immune response in 90% of cases, the duration of which varies from 2 to 10 years.
RELEASE FORM
Appearance: Solution for intramuscular administration 0.5 ml/dose.
1 dose (0.5 ml) of the drug in bottles made of transparent borosilicate glass (type 1) with a capacity of 3 ml, which are sealed with a stopper made from a mixture of chlorobutyl (latex-free) and synthetic polyisoprene, and rolled up with an aluminum cap equipped with a tear-off plastic lid "flip-off" type.
1 or 5 bottles along with instructions for medical use in a cardboard pack.
Types of Vaccines
There is no single vaccine against all meningitis pathogens. This is due to the characteristics of bacteria and viruses that trigger the infectious process.
Vaccines against meningococci
Vaccine preparations help cope with meningococci of half groups A, C, W-135, Y. The following vaccines are allowed in Russia:
- Meningococcal vaccine produced in Russia. Allows protection against meningococci serotypes A and C, but does not prevent the development of purulent meningococcal infection. Use is allowed from 1.5 years, after 3 years revaccination is required;
- Meningo A+C made in France. The drug prevents the development of cerebrospinal meningitis. Widely used in adults and children over 1.5 years old;
- Mencevax ACWY (Belgium). The drug reduces the risk of developing meningococcal infection caused by meningococcal serogroups A, C, W, Y. Use for vaccination of children over 2 years of age and adults is allowed;
- Menactra (USA). Vaccination allows you to build immunity to pathogens that are included in serogroups A, C, Y and W-135 in children over 2 years of age and adults up to 55 years of age.
Vaccines against meningococcal infection are produced in the form of a dry substance, which should be diluted immediately before administration with a solvent. The drug is administered subcutaneously or intramuscularly.
Haemophilus influenzae vaccine
The vaccine drug ACT - Hib, approved in Russia, will help prevent the development of hemophilus influenzae infection. It is created on the basis of particles of the cell wall of the pathogen. The vaccine is produced in the form of a lyophilisate – a dry powder. Immediately before administration, the drug is diluted with a solvent or other vaccine preparation. Tetracoccus is used quite often, which is aimed at creating immunity in a child to whooping cough, polio, diphtheria and tetanus.
The meningitis vaccine involves an intramuscular injection into the thigh or shoulder. The drug is well tolerated and provides reliable protection against hemophilus influenzae infection.
Vaccines against pneumococcal meningitis
The following vaccine preparations are widely used in our country:
- Pneumo 23 (France). The vaccine is given to children after 2 years of age; it allows them to create immunity for 10 years;
- Prevenar 13. The drug is used in children from 2 months to 5 years of age. For lifelong immunity, 4 injections are enough. Vaccinations are given free of charge to children who are often sick.
COMPOUND
One dose (0.5 ml) contains:
Active substances:
Monovalent meningococcal conjugates (polysaccharide + carrier protein):
- Polysaccharide serogroup A* - 4 µg;
- Polysaccharide serogroup C* - 4 mcg;
- Polysaccharide serogroup Y* - 4 mcg;
- Polysaccharide of serogroup W-135* - 4 mcg.
* - each polysaccharide is conjugated with diphtheria toxoid. The protein content of diphtheria toxoid in the vaccination dose is about 48 µg.
Excipients:
- Sodium chloride 4.35 mg;
- Sodium hydrogen phosphate 0.348 mg;
- Sodium dihydrogen phosphate monohydrate 0.352 mg;
- Water for injection - up to 0.5 ml.
Vaccination schedule
Breastfed newborns are protected from Haemophilus influenzae infection due to antibodies that come from mother's milk. Therefore, vaccinations against meningitis are carried out in children older than 3 months. The following immunization regimens are used:
- If vaccination begins at 3 months, then 3 vaccinations are indicated with an interval of 1.5 months. Revaccination is carried out at 1.5 years. Injections are usually combined with the administration of DTP and Tetracoccus vaccines;
- If vaccination starts at 6 months, then 2 vaccinations with an interval of 1.5 months are sufficient. Revaccination is carried out 12 months after the last injection;
- For children over one year old and people with immunodeficiency, the vaccine is administered once.
The Prevenar vaccine is administered to a child according to the following scheme:
- 3 months;
- 4.5 months;
- 6 months;
- Revaccination at 1.5 years.
The vaccine preparation Pneumo-23 is administered once to a child over 2 years of age.
INTERACTION WITH OTHER MEDICINES
Menactra vaccine was used simultaneously with a polysaccharide vaccine for the prevention of typhoid fever and with an adsorbed vaccine containing tetanus and diphtheria toxoids intended for use in adults (Td), in persons aged 18-55 years and 11-17 years, respectively.
In children younger than 2 years of age, Menactra was given with one or more of the following vaccines: pneumococcal conjugate vaccine (PCV), measles, mumps, rubella vaccine, varicella vaccine, or hepatitis A vaccine.
There are no data to evaluate the safety and immunogenicity of the Menactra vaccine when used together at the age of 18 months with DPT-containing vaccines.
Pneumococcal antibody titers against certain serotypes contained in the 7-valent pneumococcal conjugate vaccine (PCV7) were reduced after coadministration of Menactra and PCV7.
The BCG vaccine should not be used simultaneously with the Menactra vaccine. Vaccines must always be administered to different parts of the body, using separate syringes for each of them.
Possible adverse reactions
Meningitis vaccines are usually well tolerated. However, in some patients, vaccination provokes the following side effects:
- Weakness;
- Redness at the injection site, development of painful swelling;
- Fever in rare cases;
- Severe allergies, accompanied by swelling of the oral cavity, difficulty breathing, tachycardia, shortness of breath, pale skin, urticaria;
- Possible exacerbation of chronic diseases.
Most adverse reactions do not require special drug therapy. However, if an allergy develops, the patient needs to take an antihistamine; if symptoms are severe, an ambulance must be called.
Features of the prevention of meningitis at the site of infection
In Russia, a single dose of immunoglobulin is recommended for school-age children to prevent meningitis. The injection must be given within a week after contact with a patient or carrier of the infection. To prevent the occurrence of secondary meningitis, it is recommended to vaccinate a child within 5 days after contact with infected people.
At the site of infection, it is also recommended to follow the following general recommendations:
- Avoid swimming in unknown bodies of water;
- Avoid large crowds of people;
- Wash your hands with soap before eating, after walking, traveling on public transport;
- Regularly carry out wet cleaning of premises;
- Drink only high-quality drinking water;
- Carefully process products before consumption.
Do children need vaccinations?
Parents must decide for themselves whether their child needs vaccination. To make the right choice, you need to consider:
- If someone from the child’s environment gets meningitis, then the risk of infection of the baby can reach 95%;
- In countries with mandatory immunization, the development of meningitis has been almost completely eliminated;
- Vaccines are highly effective and well tolerated;
- Vaccination is necessary before traveling to dangerous meningitis areas;
- The first symptoms of meningitis may resemble a common cold, so it can be difficult to recognize the infection in a timely manner.
Each parent decides independently whether their child needs vaccination against meningitis. In Russia, vaccination is not included in the routine vaccination calendar. Therefore, parents need to buy the vaccine themselves. The World Health Organization recommends that all children be immunized. This will reliably protect the child from a fatal infection.