Pharmacological properties of the drug Remeron
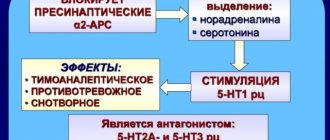
Pharmacodynamics. Mirtazapine is an antagonist of presynaptic α2 receptors in the central nervous system and enhances central noradrenergic and serotonergic transmission of nerve impulses. Enhancement of serotonergic transmission occurs exclusively through 5-HT1 receptors, since mirtazapine blocks 5-HT2 and 5-HT3 receptors. Both spatial enantiomers of mirtazapine exhibit antidepressant activity, with the S(+) enantiomer blocking α2 and 5-HT2 receptors, and the R(-) enantiomer blocking 5-HT3 receptors. In addition, mirtazapine blocks H1 receptors, which determines its sedative properties. In therapeutic doses, mirtazapine exhibits virtually no anticholinergic activity and does not affect the cardiovascular system. Pharmacokinetics. After oral administration, mirtazapine is rapidly absorbed. Bioavailability is approximately 50%. The maximum plasma concentration is observed after approximately 2 hours. Approximately 85% of mirtazapine is bound to plasma proteins. The half-life averages 20–40 hours; in some cases - up to 65 hours. Usually, in young people, the half-life is shorter than in older people. The long half-life allows the drug to be taken once a day. The equilibrium concentration of the active substance in the blood plasma is achieved after 3–4 days; thereafter it does not change. In the range of recommended doses, the pharmacokinetic parameters of mirtazapine have a linear dependence on the dose. Mirtazapine is actively metabolized and excreted from the body in urine and feces over several days. The main routes of its metabolism in the body are demethylation and oxidation with further conjugation. Demethylmirtazapine is also pharmacologically active. The clearance of mirtazapine may be reduced in patients with renal or hepatic impairment.
Overdose
Available information regarding Remeron overdose suggests that symptoms are usually mild. There are reports of central nervous system depression, which was expressed in prolonged sedation and spatial disorientation in combination with a slight increase or decrease in blood pressure and tachycardia. However, taking a dose much higher than the therapeutic dose (especially in overdose caused by simultaneous use of several drugs) may carry a risk of more serious consequences, including death. In such cases, symptoms such as tachycardia of the “torsades de pointes” type and prolongation of the QT interval are observed.
In case of overdose, symptomatic therapy is prescribed to ensure the maintenance of vital body functions, and cardiac activity is regularly monitored using an ECG. It is also recommended to administer activated charcoal or perform gastric lavage.
Use of the drug Remeron
Remeron is used once a day, preferably at night before bedtime. Remeron can also be divided into two doses (once in the morning and once in the evening, with the larger dose taken at night). The tablets should be taken orally, swallowed without chewing, and, if necessary, washed down with water. Patients with depression should be treated for a long time, at least 6 months, until symptoms disappear completely. It is recommended that mirtazapine treatment be discontinued gradually to avoid withdrawal symptoms. Adults The effective daily dose is usually 15–45 mg; initial dose 15 or 30 mg. If the initial dose is 15 mg, and the daily dose is 15 or 45 mg, tablets of the appropriate dosage are used - 15 or 45 mg. Mirtazapine begins to show effects after 1–2 weeks of treatment. Treatment with an adequate dose causes a positive response within 2–4 weeks. If the response is insufficient, the dose can be increased. If there is no response within the next 2–4 weeks, the drug should be discontinued. If it is necessary to use a dose of 15 mg and 45 mg, a different dosage form with the appropriate dosage should be used. Elderly patients The recommended dose is the same as for adults. In order to achieve a satisfactory and safe result, increasing the dose for elderly patients is carried out under the strict supervision of a physician. Renal impairment Mirtazapine clearance may be reduced in patients with moderate or severe renal impairment (creatinine clearance ≤40 ml/min). When prescribing Remeron to this category of patients, it is necessary to monitor creatinine clearance. Hepatic impairment The clearance of mirtazapine may be reduced in patients with hepatic impairment. This fact should be taken into account when prescribing Remeron to this category of patients, especially those with severe liver failure, since such patients have not been studied. Prescribe Remeron, starting with the minimum dose and monitoring the clearance of mirtazapine, especially if the dose is increased.
Consequences of drinking alcohol
If you mix antidepressants and alcohol, the effects can appear instantly, or they can occur with a delay of 1-2 hours. The outcome largely depends on concomitant factors, the body, the amount of drink consumed and the duration of therapy. The quality of alcohol and the availability of snacks also have a special influence.
The most likely consequences of the symbiosis of two substances:
- increased heart rate (up to 120 beats per minute);
- liver damage;
- hallucinations;
- development of depressive psychosis and sexual disorders;
- the risk of sudden changes in pressure increases;
- heart rhythm disturbance;
- insufficient or increased blood clotting.
Doctors and drug manufacturers warn buyers about possible complications of combining an antidepressant and alcohol. Such information is indicated in the instructions for use attached to the medication.
Side effects of the drug Remeron
Patients with depression exhibited symptoms that may be related to the disease itself. However, it is difficult to determine which symptoms are the result of the disease and which are the result of treatment with Remeron. The most common adverse reactions, occurring in more than 5% of patients treated with Remeron in randomized placebo-controlled trials (see below), are drowsiness, sedation, dry mouth, weight gain, increased appetite, dizziness, and increased fatigue. All randomized placebo-controlled studies of patients (including indications other than major depressive disorder) assessed the side effects of Remeron. The meta-analysis included 20 studies with a planned treatment duration of up to 12 weeks, with 1501 patients (134 patient-years) receiving mirtazapine doses up to 60 mg and 850 patients (79 patient-years) receiving placebo. The extension phases of these studies were excluded to maintain comparability with placebo treatment. Table 1 shows the incidence of adverse reactions that occurred in clinical studies. Frequently, adverse reactions were more statistically significant during treatment with Remeron than with placebo, given spontaneous reports of adverse reactions. Spontaneous adverse reactions that were reported from randomized placebo-controlled trials in patients who did not experience any events associated with mirtazapine were classified as “unknown.” Table 1. Adverse reactions of the drug Remeron.
Organ systems | Very common (≥1/10) | Frequent (≥1/100 to ≤1/10) | Uncommon (≥1/1000 to ≤1/100) | Rare (≥1/10000 to ≤1/1000) | Frequency unknown |
| General manifestations | Weight gain 1 | ||||
| Blood and lymph systems | Bone marrow suppression (granulocytopenia, agranulocytosis, aplastic anemia, thrombocytopenia) Eosinophilia | ||||
| CNS | Drowsiness 1, 4 Sedation 1, 4 Headache 2 | Lethargy 1 Dizziness Tremor | Paresthesia 2 Leg fatigue Syncope | Myoclonus | Convulsions (hemorrhages) Serotonin syndrome Paresthesia of the oral mucosa |
| Gastrointestinal tract | Dry mouth | Nausea 3 Diarrhea 2 Vomiting 2 | Oral hyposthesia | Swelling of the oral mucosa | |
| Skin and subcutaneous tissue | Exanthema2 | ||||
| Musculoskeletal system | Arthralgia Myalgia Back pain 1 | ||||
| Endocrine system | Impaired secretion of antidiuretic hormone | ||||
| Metabolism | Increased appetite 1 | Hyponatremia | |||
| Vascular manifestations | Orthostatic hypotension | Arterial hypotension 2 | |||
| General manifestations and reactions at the injection site | Peripheral edema 1 Increased fatigue | ||||
| Hepatobiliary system | Increased activity of plasma transaminases | ||||
| Mental manifestations | Sleep disorders Confusion Anxiety 2.5 Insomnia 3.5 | Nightmares 2 Mania Agitation 2 Hallucinations Psychomotor agitation (including akathisia, hyperkinesia) | Suicidal thoughts 6 Suicidal behavior 6 |
1 These adverse reactions occurred with greater frequency during treatment with Remeron than placebo in clinical studies. 2 These adverse reactions occurred at a higher frequency during treatment with placebo than with Remeron in clinical studies, but were not statistically significant. 3 These adverse reactions occurred with greater frequency during treatment with placebo than with Remeron in clinical studies. 4 NB Dose reduction does not usually reduce drowsiness/sedation, but may compromise the effectiveness of the antidepressant. 5 Agitation and insomnia (which may be symptoms of depression) may develop or worsen as a result of treatment with antidepressants. Development or worsening of agitation and insomnia have been reported during treatment with mirtazapine. 6 Cases of suicidal ideation and suicidal behavior have been reported during mirtazapine therapy or immediately after discontinuation of treatment.
When evaluating the data that were obtained during clinical studies, transaminase and gamma-glutamyl transferase were temporarily increased (however, adverse reactions associated with Remeron were not reported, which occurred with a higher frequency during treatment with Remeron than placebo).
Special instructions for the use of the drug Remeron
Suicide/suicidal thoughts or worsening clinical symptoms. Depression is associated with an increased risk of suicidal ideation, self-harm, or suicide. The risk may last until significant remission occurs. Since improvement may not occur for the first few weeks of treatment or more, patients should be closely monitored until such improvement occurs. It is common clinical experience that the risk of suicide may increase in the early stages of recovery. It is known that patients with a history of suicidal events, or patients who exhibit a significant degree of suicidal ideation even before treatment, have a higher risk of suicidal ideation or suicide attempts, and should be closely monitored during treatment. A meta-analysis of placebo-controlled clinical trials of antidepressants used in adults with mental disorders showed an increased risk of suicidal behavior with antidepressants compared with patients under 25 years of age who received placebo. Careful monitoring of patients, especially those at high risk for suicidal behavior, should be accompanied by antidepressant therapy, especially during initial therapy and after dosage changes. Patients (and caregivers of patients) should be warned to be alert to any clinical signs, suicidal behavior or thoughts, and unusual changes in behavior, and to seek medical advice immediately if such symptoms are present. Taking into account the possibility of suicide, especially at the beginning of treatment, the patient should be given only the minimum required number of Remeron tablets. Suppression of bone marrow function. Bone marrow depression, usually manifested by granulocytopenia or agranulocytosis, has been reported during treatment with Remeron. Reversible agranulocytosis has been reported as a rare occurrence in clinical studies of Remeron. In the post-marketing period, very rare cases of agranulocytosis have been reported, most cases reversible, but in some cases fatal. Deaths occurred in patients over 65 years of age. Your doctor should watch for symptoms such as fever, sore throat, stomatitis, or other signs of infection. When such symptoms occur, treatment should be stopped and a clinical blood test performed. Jaundice Treatment should be stopped if jaundice occurs. Conditions that require medical supervision. Careful dosing and regular and close monitoring are necessary for patients with the following conditions:
- epilepsy and organic brain lesions: although clinical experience shows that epileptic seizures occur rarely during treatment with mirtazapine, as with treatment with other antidepressants, Remeron should be used with particular caution in patients with a history of epileptic seizures. In patients who develop epileptic seizures, or when there is an increase in the frequency of epileptic seizures, treatment should be discontinued;
- Hepatic Impairment: Following a 15 mg oral dose of mirtazapine, the clearance of mirtazapine is reduced by approximately 35% in patients with mild to moderate hepatic impairment compared with patients with normal hepatic function. The average plasma concentration of mirtazapine increases by approximately 55%. When prescribing 30 mg of mirtazapine, it is necessary to take into account the benefit/potential risk ratio for the patient;
- Renal impairment: After a single 15 mg oral dose of mirtazapine in patients with moderate (creatinine clearance ≤40 mL/min - 10 mL/min) or severe (creatinine clearance ≤10 mL/min) renal impairment, mirtazapine clearance decreased by approximately 30 % and 50%, respectively, compared to healthy patients. The mean plasma concentrations of mirtazapine increased by 55% and 115%, respectively. There were no significant differences observed in patients with mild renal impairment (creatinine clearance ≤80 mL/min to 40 mL/min) compared with controls. When prescribing 30 mg of mirtazapine, it is necessary to consider the benefit/potential risk ratio for the patient and monitor creatinine clearance;
- heart diseases such as conduction disorders, angina pectoris and recent myocardial infarction. Such cases require the usual precautions and concomitant therapy with caution;
- arterial hypotension;
- Diabetes mellitus: In patients with diabetes, antidepressants may affect blood glucose levels. Dosage adjustments of insulin and/or oral hypoglycemic agents may be necessary and close monitoring is recommended.
As with other antidepressants, the following must be taken into account:
- when taking antidepressants in patients with schizophrenia or other mental disorders, psychotic symptoms may worsen; Paranoid thoughts may become more intense;
- When treating the depressive phase of bipolar disorder, it can transform into a manic phase. Patients with a history of manic or hypomanic symptoms should be carefully monitored. Mirtazapine should be discontinued if the patient enters a manic phase;
- Although Remeron is not addictive, post-marketing experience suggests that sudden cessation of treatment after prolonged use may sometimes lead to withdrawal symptoms. Most withdrawal reactions are clinically mild and resolve on their own. Among the variety of withdrawal symptoms reported, the most common were dizziness, agitation, restlessness, headache and nausea. Although these have been reported as withdrawal symptoms, it is important to understand that these symptoms may be related to the course of the underlying medical condition. It is recommended to gradually discontinue treatment with mirtazapine;
- Caution is required when treating patients with urinary dysfunction, incl. with prostatic hypertrophy, patients with acute angle-closure glaucoma and increased intraocular pressure (however, the effect of Remeron is unlikely due to very weak anticholinergic activity);
- akathisia/psychomotor agitation: the use of antidepressants is associated with the development of akathisia, which is characterized by subjectively unpleasant or anxious agitation with increased motor activity. It is most likely that these symptoms may occur during the first few weeks of treatment, so increasing the dose may be harmful to health.
Hyponatremia. Hyponatremia, which is associated with inappropriate antidiuretic hormone (ADH) secretion, has been very rarely reported with mirtazapine. Elderly patients or patients taking concomitant medications known to cause hyponatremia require the use of precautions. Serotonin syndrome. Interaction with serotonergic active substances: Serotonin syndrome may occur when selective serotonin reuptake inhibitors are used together with other serotonergic active substances. Symptoms of serotonin syndrome may include hyperthermia, rigidity, myoclonus, and autonomic instability with possible rapid fluctuations in vital signs. Mental status changes include confusion, irritability, and extreme agitation that progress to delirium and coma. It is known from post-marketing experience that serotonin syndrome occurs very rarely in patients who use only Remeron. Elderly patients When prescribing Remeron to elderly patients, it is necessary to take into account undesirable effects when using antidepressants. In clinical studies of the drug Remeron, the occurrence of adverse reactions in elderly patients was observed no more often than in patients of other age groups. Lactose The drug contains lactose. Patients with rare hereditary diseases of galactose intolerance, Lapp-lactase deficiency or glucose-galactose malabsorption are contraindicated to take the drug. Use of the drug during pregnancy and lactation. Limited data from the use of mirtazapine in pregnant women do not indicate an increased risk of birth defects. Animal studies have not shown any teratogenic effects that manifest as clinical symptoms, but adverse effects on intrauterine development have been observed. Caution is necessary, taking into account the benefit/potential risk ratio for the fetus, when prescribing the drug to pregnant women. If Remeron is used before or immediately before birth, postnatal monitoring of the newborn is recommended to account for possible withdrawal effects. Animal studies and the limited available data from human studies have shown very low levels of mirtazapine excreting in breast milk. The decision whether to continue/discontinue breastfeeding or continue/discontinue therapy with Remeron should be made taking into account the benefits to the baby and the benefits of therapy with Remeron to the woman. Children. Remeron is not used to treat patients under 18 years of age. Behaviors related to suicide (suicide attempts and suicidal ideation) and aggression (primarily aggression, negativism, and anger) were most frequently observed in clinical studies among children and adolescents treated with antidepressants compared with children and adolescents treated with placebo. If a decision to treat is made based on clinical need, the patient should be closely monitored for the emergence of suicidal symptoms. In addition, long-term safety data in children and adolescents regarding growth, maturation, cognitive and behavioral development are lacking. Effect on the ability to drive a car and other mechanisms Remeron affects concentration. Patients taking antidepressants should not engage in potentially hazardous activities, including driving a car or using other machinery.
Contraindications
"Remeron" is a drug that cannot be used by people who are hypersensitive to its main active component - the substance mirtazapine, as well as to any of the auxiliary components of the drug. The following contraindications are pregnancy, lactation, renal or liver failure. There are also relative contraindications:
- prostatic hypertrophy;
- diabetes;
- angle-closure glaucoma;
- atrial fibrillation;
- arterial hypotension;
- angina pectoris;
- organic lesions of the brain (brain);
- tendency to abuse psychoactive drugs.
Drug interactions Remeron
Pharmacodynamic interactions.
- Mirtazapine should not be taken concomitantly with MAO inhibitors or within two weeks after the end of therapy. Conversely, approximately two weeks must pass before patients treated with mirtazapine begin taking MAO inhibitors.
- Concomitant use with other serotonergic active substances (L-tryptophan, triptan, tramadol, linezolid, selective serotonin reuptake inhibitors, venlafaxine, lithium and preparations with St. John's wort ( Hypericum perforatum ) may lead to serotonin-related effects, which requires special caution when purpose;
- Mirtazapine may enhance the sedative properties of benzodiazepines and other sedatives (in particular, most antipsychotics, antihistamine H1 antagonists, opioids). Caution should be used when prescribing these drugs with mirtazapine;
- Remeron may enhance the depressive effect of alcohol on the central nervous system. Therefore, patients should be warned not to consume alcoholic beverages while taking mirtazapine;
- Mirtazapine at doses of 30 mg once daily caused a small but statistically significant increase in the international normalized index (INR) in patients receiving warfarin. It is recommended to monitor MNI in case of concomitant treatment with warfarin and mirtazapine due to a possible increase in MNI.
Pharmacokinetic interactions
- Carbamazepine and phenytoin, inducers of CYP3A4, increase the clearance of mirtazapine by approximately 2 times, and, as a result, the average concentration of mirtazapine in the blood plasma decreases by 60% and 45%, respectively. When carbamazepine or any other inducer of hepatic metabolism (such as rifampicin) is added to mirtazapine therapy, the dose of mirtazapine must be increased. If treatment with this drug is discontinued, the dose of mirtazapine may need to be reduced;
- concomitant administration of the strong CYP3A4 inhibitor ketoconazole increased peak plasma levels and AUC of mirtazapine by approximately 40% and 50%, respectively;
- when cimetidine (a weak inhibitor of CYP1A2, CYP2D6 and CYP3A4) is administered with mirtazapine, mean plasma concentrations of mirtazapine may increase by more than 50%. Precautions and dose reductions should be taken when mirtazapine is administered with strong CYP3A4 inhibitors, HIV protease inhibitors, azole antifungals, erythromycin, cimetidine, or nefazodone;
- interaction studies did not reveal any relevant pharmacokinetic effects when mirtazapine was co-treated with paroxetine, amitriptyline, risperidone or lithium.
Composition of the medicine
The product is available in tablet form.
The tablets have a white shell. Available in dosages of 15, 30 and 45 milligrams. The tablets are packaged in blisters of ten pieces. Blisters are placed in cardboard packaging, one or three pieces each.
The active ingredient in Remeron is mirtazapine. Depending on the dosage, each tablet contains 45, 30 or 15 milligrams of the active substance.
The auxiliary components of the product include:
- corn starch;
- magnesium stearate;
- hyprolose;
- colloidal silicon dioxide;
- lactose monohydrate.