Paroxetine is a well-studied psychotropic drug and belongs to the new generation of antidepressants. This drug belongs to the group of selective serotonin reactivation inhibitors.
Paroxetine is characterized by the fact that it quickly eliminates the symptoms of depression and has an anxiolytic (anxiety-relieving) effect.
The medicine is a highly effective neurostimulant and has a wide spectrum of antidepressant activity. The medication has quite wide application in psychiatry. This psychotropic drug is tolerable and has a low number of side effects.
Indications for use
Prescribed in therapy:
- depressive states;
- social phobias;
- panic disorders;
- obsessive-compulsive disorders;
- post-traumatic stress conditions;
- generalized anxiety disorders.
According to numerous clinical studies, Paroxetine tablets are effective in monotherapy. In most cases, additional drug support is not required.
Notes
- Trends in Drug Patenting - Case Studies: THE CASES: 1. PAROXETINE // WHO, 2001. Retrieved March 16, 2012. Archived June 24, 2012.
- WHO | Paroxetine hydrochloride (Inclusion). Essential medicines selection, 17th Expert Committee on the Selection and Use of Essential Medicines (2009). Retrieved March 16, 2012. Archived June 24, 2012.
- ↑ 12345
Paroxetine // Medicines / M. D. Mashkovsky. — Mashkovsky’s directory on-line. - Marks DM, Park MH, Ham BJ, Han C, Patkar AA, Masand PS, Pae CU. (November 2008). "Paroxetine: safety and tolerance issues." Expert Opin Drug Saf 7
(6):783–94. PMID 18983224. - ↑ 1 2
Bourin M, Chue P, Guillon Y. (Spring 2001).
"Paroxetine: a review." CNS Drug Rev 7
(1):25–47. PMID 11420571. - ↑ 1234567891011
PAXIL (paroxetine hydrochloride) Tablets and Oral Suspension: PRESCRIBING INFORMATION (PDF). Research Triangle Park, NC: GlaxoSmithKline (August 2007). Retrieved August 14, 2007. Archived August 25, 2011. - Roth, B.L.;
Driscol, J. PDSP Ki Database.
Psychoactive Drug Screening Program (PDSP)
. University of North Carolina at Chapel Hill and the United States National Institute of Mental Health (January 12, 2011). Retrieved November 22, 2013. - Brunton, L;
Chabner, B; Knollman, B. Goodman and Gilman's The Pharmacological Basis of Therapeutics. — 12th. - New York: McGraw-Hill Professional, 2010. - ISBN 978-0-07-162442-8. - Drobizhev M.Yu., Mukhin A.A.
Selective serotonin reuptake inhibitors: options for choice (comments on the works of Thase et al.) // Psychiatry and psychopharmacotherapy. - 2004. - T. 6, No. 1. - Ushkalova E.A., Ushkalova A.V.
Pharmacotherapy of depression in cardiac patients // Difficult Patient. - 2006. - No. 1. - ↑ 1 2
Aursnes I, Gjertsen MK.
(July 2008). "Common adverse events associated with an SSRI: meta-analysis of early paroxetine data." Pharmacoepidemiol Drug Saf 17
(7):707–13. PMID 18383561. - ↑ 1 2 3 4 5 Podkorytov V. S., Chaika Yu. Yu.
Depression. Modern therapy. - Kharkov: Tornado, 2003. - 352 p. — ISBN 966-635-495-0. - Grimsley SR, Jann MW. (November 1992). "Paroxetine, sertraline, and fluvoxamine: new selective serotonin reuptake inhibitors." Clin Pharm 11
(11):930–57. PMID 1464219. - ↑ 1 2 3 4 5 Gubsky Yu. I., Shapovalova V. A., Kutko I. I., Shapovalov V. V.
Medicines in psychopharmacology. - Kyiv - Kharkov: Health - Torsing, 1997. - 288 p. — 20,000 copies. — ISBN 5-311-00922-5, 966-7300-04-8. - Baldassano CF, Truman CJ, Nierenberg A, Ghaemi SN, Sachs GS (March-April 1996). "Akathisia: a review and case report following paroxetine treatment." Compr Psychiatry 37
(2):122–4. PMID 8654061. - Ener RA, Meglathery SB, Van Decker WA, Gallagher RM
Serotonin syndrome and other serotonergic disorders. (English) // Pain medicine (Malden, Mass.). - 2003. - Vol. 4, no. 1. - P. 63-74. - PMID 12873279. [correct] - Torre D, Falorni A.
Pharmacological causes of hyperprolactinemia // Ther Clin Risk Manag. - Oct 2007. - T. 3, No. 5. - P. 929-951. - Papakostas GI. (2008). "Tolerance of modern antidepressants". J Clin Psychiatry 69
(E1):8–13. PMID 18494538. - Colakoglu O, Tankurt E, Unsal B, Ugur F, Kupelioglu A, Buyrac Z, Akpinar Z (July 2005). "Toxic hepatitis associated with paroxetine." Int J Clin Pract 59
(7):861–2. PMID 15963219. - Guzmán Ruiz O, Ramírez Martín del Campo M, Fernandez López I, Romero Gómez M (March 2005). "Hepatotoxicity induced by paroxetine." Med Clin (Barc) 19
(124(10)): 399. PMID 15766517. - ↑ 1 2 Aursnes I., Tvete IF, Gaasemyr J., Natvig B.
Even more suicide attempts in clinical trials with paroxetine randomized against placebo. (English) // BMC psychiatry. - 2006. - Vol. 6. - P. 55. - DOI:10.1186/1471-244X-6-55. - PMID 17129393. [correct] - ↑ 123
Paxil (paroxetine hydrochloride) Tablets and Oral Suspension. FDA (05/12/2006). - Tang SW, Helmeste D. (April 2008). "Paroxetine". Expert Opin Pharmacother 9
(5):787–94. PMID 18345955. - Bauer MS, Wisniewski SR, Kogan JN, Marangell LB, Thase ME, Sachs G. (2006). "Brief report: paroxetine in younger and adult individuals at high risk for suicide." Psychopharmacol Bull 39
(1):31–7. PMID 17065973. - ↑ 1 2
Barbui C, Furukawa TA, Cipriani A (January 2008).
"Effectiveness of paroxetine in the treatment of acute major depression in adults: a systematic re-examination of published and unpublished data from randomized trials." CMAJ 178
(3):296–305. DOI:10.1503/cmaj.070693. PMID 18227449. - Paroxetine (Paxil) and fluoxetine (Prozac) can cause fetal development disorders // Moscow Regional Psychiatric Newspaper. — January — February 2009 — No. 1 (45).
- Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB (July 1998). "Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial." Biol. Psychiatry 44
(2):77–87. PMID 9646889. - ↑ 1 2
Black K, Shea C, Dursun S, Kutcher S (May 2000).
"Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria." J Psychiatry Neurosci 25
(3):255–61. PMID 10863885. - Belloeuf L, Le Jeunne C, Hugues FC (April 2000). "Paroxetine withdrawal syndrome". Ann Med Interne (Paris) 151
(124(10)): 399. PMID 10855379. - Renoir T.
Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved. (English) // Frontiers in pharmacology. — 2013. — Vol. 4. - P. 45. - DOI:10.3389/fphar.2013.00045. - PMID 23596418. [correct] - ↑ 12
Reference Guide to Psychopharmacological and Antiepileptic Drugs Approved for Use in Russia / Ed. S. N. Mosolova. — Ed. 2nd, revised - M.: BINOM Publishing House, 2004. - P. 139-140. — 304 p. — 7000 copies. — ISBN 5-9518-0093-5. - Puzhinsky S.
Pharmacotherapy of depressive states // Depression and comorbid disorders / Ed. Smulevich A.B. - M., 1997. - Hammad TA, Laughren T, Racoosin J (March 2006). "Suicidality in pediatric patients treated with antidepressant drugs." Arch. Gen. Psychiatry 63
(3):332–9. DOI:10.1001/archpsyc.63.3.332. PMID 16520440. - Report of the CSM expert working group on the safety of selective serotonin reuptake inhibitor antidepressants (PDF). MHRA (December 2004). Retrieved February 17, 2009. Archived August 25, 2011.
- Glaxo releases classified materials
- Thormahlen GM (October 2006). "Paroxetine use during pregnancy: is it safe?". Ann Pharmacother 40
(10):1834–7. PMID 16926304. - ↑ 12345
Rational pharmacotherapy in psychiatric practice: a guide for practitioners / Ed. ed. Yu. A. Alexandrovsky, N. G. Neznanov. - Moscow: Litterra, 2014. - 1080 p. — (Rational pharmacotherapy). — ISBN 978-5-4235-0134-1. - Malin D.I.
Drug interactions of psychotropic drugs (Part II) // Psychiatry and psychopharmacotherapy. - 2000. - ↑ 1 2 Minutko V.L.
Depression. - Moscow: GEOTAR-Media, 2006. - 320 p. — 2000 copies. — ISBN 5-9704-0205-2. - For 15 years, a large pharmaceutical company hid data on the relationship between an increased risk of suicide and the use of its antidepressant (February 13, 2008). Source: New Scientist magazine, 06 February 2008, page 12.
- ↑ 1 2 3 Goetsche P.
Deadly drugs and organized crime: How Big Pharma corrupted healthcare / [Trans. from English L. E. Ziganshina]. - Moscow: Publishing House "E", 2021. - 464 p. — (Evidence-based medicine). — 3000 copies. — ISBN 978-5-699-83580-5. - Healy D, Herxheimer A, Menkes DB.
Antidepressants and Violence: Problems at the Interface of Medicine and Law // PLoS Med. - 2006. - T. 3, No. 9. - DOI:10.1371/journal.pmed.0030372. - The secrets of seroxat // BBC News / Programs / Panorama. See more details: https://news.bbc.co.uk/2/shared/spl/hi/programmes/panorama/transcripts/seroxat.txt
- Angell M.
The Epidemic of Mental Illness: Why?. The New York Review of Books (June 23, 2011). Archived from the original on September 21, 2012. - Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (February 2008). "Initial Severity and Antidepressant Benefits: A Meta-Analysis of Data Submitted to the Food and Drug Administration." PLoS Medicine 5
(2):e45. DOI:10.1371/journal.pmed.0050045. PMID 18303940. - Horder J, Matthews P, Waldmann R (June 2010). "Placebo, Prozac and PLoS: significant lessons for psychopharmacology." Journal of Psychopharmacology 25
(10): 1277–88. DOI:10.1177/0269881110372544. PMID 20571143. - McGauran N, Wieseler B, Kreis J, Schüler YB, Kölsch H, Kaiser T. (2010 Apr 13). “Reporting bias in medical research - a narrative review.” Trials 11
: 37. DOI:10.1186/1745-6215-11-37. PMID 20388211. - Goldacre B.
The whole truth about drugs: The global conspiracy of pharmaceutical companies / Translation: Poroshina T.I., Cherepanov V.V. — RIPOL Classic, 2015. — 630 p. — (New way of life).
Instructions for use
Taken orally after eating.
Recommended administration time is evening. It is prescribed once a day.
At the initial stage of therapy - 10-20 mg. Gradually the dosage can be increased to 50 mg.
In the first 2-3 weeks, the doctor selects the dosage that is optimal for the patient. The duration of the therapeutic course is up to 3 months.
It is not advisable to abruptly stop taking an antidepressant. To avoid the development of “withdrawal syndrome,” the dose of the drug should be gradually reduced.
Pharmacological profile
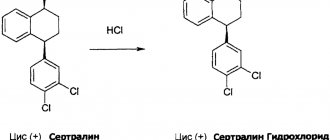
Structure of paroxetine hydrochloride hemihydrate
The drug has an antidepressant effect.
The structure of the drug is bicyclic, which distinguishes it from other similar drugs.
The antidepressant effect of this drug is quite high; a positive result may appear already on the third day after the start of its use, and after 2-3 weeks Paroxetine relieves the symptoms of panic disorder and anxiety.
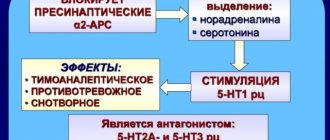
The action of the drug is associated with blocking the uptake of serotonin, which leads to an increase in its activity in the central nervous system.
Serotonin is one of fifty neurotransmitters that are produced in the brain. They are responsible for connecting billions of nerve cells in the brain with each other. The following body functions depend on the activity of serotonin: sleep, behavior, mood, sexual desire.
Many forms of depression are associated precisely with a lack of serotonin in the brain. Paroxetine effectively affects the level of this mediator in the central nervous system.
It acts quite gently, without disturbing psychomotor functions, and does not lead to significant changes in blood pressure and heart rate. The anticholinergic effect of this antidepressant leads to a rapid reduction of insomnia and anxiety.
Restrictions
It is prohibited to prescribe an antidepressant for:
angle-closure glaucoma;- prostatic hyperplasia;
- severe manic state;
- heart pathologies;
- epileptic seizures;
- convulsive states;
- diseases that increase the risk of bleeding;
- renal/liver pathologies.
It is not recommended to prescribe the drug to elderly patients.
Side effects
An antidepressant can cause many adverse reactions:
- migraine;
- dizziness;
- drowsiness during the day, night insomnia;
- aggression;
- anxiety, nervousness;
- disorientation;
- hallucinations;
- state of euphoria;
- impaired concentration;
- convulsions;
- persecution mania;
- disturbances in the functioning of the stomach and intestines;
- blurred vision;
- violation of taste buds;
- arthralgia;
- myopathy;
- decreased libido;
- sexual dysfunction;
- frequent urination;
- nausea;
- vomiting;
- decreased or increased appetite;
- hepatitis;
- allergic reactions;
- increased sweating.
Many patients report an increase/decrease in body weight when taking an antidepressant.
Overdose symptoms
When taking increased doses of the drug, there is a high risk of overdose, which is accompanied by:
- nausea;
- vomiting;
- severe dry mouth;
- drowsiness during the day;
- irritability, aggressiveness;
- convulsions;
- rapid heartbeat;
- dilation of the pupils.
If symptoms of overdose are detected, it is necessary to perform gastric lavage and induce vomiting to remove the undissolved part of the tablets from the stomach.
If necessary, symptomatic therapy is prescribed.
Contraindications
Concomitant treatment with MAO inhibitors[14], manic states[31], poisoning with alcohol, psychotropic drugs and other drugs[32], renal, liver failure, breastfeeding period, hypersensitivity to the drug, attack of glaucoma, age less than 15 years[12] .
It is not recommended for use in pediatric practice due to the high risk of undesirable clinical outcomes, including self-aggressive behavior[33][34], the occurrence of severe aggressiveness and increased depression[35], as well as for use during pregnancy and conception due to the risk of congenital malformations[36].
Prescription for pregnant and lactating women
During pregnancy, Paroxetine tablets can be prescribed only if there is a real threat to the woman’s mental health.
It is advisable to replace Paroxetine with a safer drug.
During therapy, it is necessary to avoid breastfeeding, since the active substance of the drug penetrates into breast milk in high concentrations and can harm the baby.
Release form and composition of the drug
The medicine is available in the form of tablets, which are coated with a film.
Each tablet is round in shape, with a radius of 0.8 mm. It is convex, has a rough surface, and is divided in the middle by a line. The main active ingredient of the drug is paroxetine hydrochloride hemihydrate in the amount of 22.76 mg.
Additional substances of this product are:
- calcium hydrogen phosphate;
- elements of microcrystalline cellulose;
- magnesium stearate;
- crospovidone;
- copovidone;
- talc.
The tablet shell consists of hypromellose, macrogol 6000, titanium dioxide.
The tablets are packaged in cardboard boxes, each blister contains 10 pieces. There may be packages of 3 blisters and 6 plates.
special instructions
For patients with suicidal tendencies, constant monitoring by doctors or loved ones is necessary. Paroxetine tablets can aggravate the patient's condition.
It is prohibited to combine with monoamine oxidase inhibitors.
Taking Paroxetine is permissible no earlier than 2 weeks after discontinuation of MAO inhibitors. If this recommendation is ignored, severe panic conditions may occur.
Daytime use of Paroxetine may cause drowsiness and powerful sedation. If such symptoms appear at the initial stage of therapy, it is recommended to take tablets only in the evening.
According to statistics, 10% of patients taking Paroxetine tablets have decreased isoenzyme activity, which is a genetic disorder.
This helps to slow down the metabolism of the active substance of the drug and the appearance of a wide range of adverse reactions. In this case, a dose adjustment of Paroxetine is necessary (a minimum amount of the drug is recommended).
It is unacceptable to combine Paroxetine with alcohol-containing drinks . There is a high probability of adverse reactions occurring in an intensified form. There are known cases of deaths when combining an antidepressant and alcohol.
During the therapeutic course, it is recommended to refrain from driving vehicles and working at hazardous sites.
Pharmacokinetics of the drug
After taking the medicine, it is quickly absorbed from the digestive tract and metabolized through the liver. Then the active component of the drug spreads between plasma and red blood cells. The medication can be taken with food, this does not affect the absorption of the active substance.
The maximum concentration of the drug in the blood after a single dose occurs after 3-10 hours. The connection of the main substance with proteins is quite high - up to 95%. A stable concentration of this medication in the body occurs after 1-2 weeks of use.
Paroxetine is half-released from plasma in 16-21 hours, which makes it possible to prescribe the medication once a day. This admission system is very suitable for patients undergoing outpatient treatment.
In inpatient settings, this allows medical staff to save time. With high doses and long-term treatment, a nonlinear accumulation of the substance in the body is observed. In patients with liver failure, the proportion of antidepressant in the blood increases.
64% of the drug is excreted in the urine, the rest is excreted through the intestines with bile.
Reviews from patients who took the drug
Real reviews from people who have undergone Paroxetine therapy:
Anton, 32 years old, Samara:
Six months ago, after suffering from stress, panic attacks, anxiety, and neurosis began. The result is a deep depression. The psychotherapist prescribed Paroxetine tablets. Sticked to therapy for 11 days. During this time, my condition only worsened. I stopped experiencing any emotions, not only negative, but also positive. I lost my appetite, as a result of which I lost 6 kilograms. Then problems with sleep began, which turned into insomnia. I felt not like a person, but a zombie.
I stopped taking the drug very abruptly, because of this I felt all the delights of “withdrawal syndrome”. In addition, during routine diagnostics it turned out that problems with the liver had begun.
I was forced to switch to a lighter herbal-based sedative.
The drug is certainly effective and helps to cope with the main problem. But it has a lot of negative sides.[/su_note]
Angelika, 25 years old, Astrakhan:
I took a Paroxetine tablet daily during postpartum depression. The course was long, 3 months. The drug is effective and helped me cope with my problem.
But it is accompanied by adverse reactions (drowsiness during the day, mild apathy, periodic dizziness, loss of appetite). It is very important to end therapy smoothly, slowly reducing the dosage. With such laughter, the body does not experience a stressful state, and without consequences you wean yourself off living without antidepressants.
More than 60% of patients complain of adverse reactions when taking Paroxetine. If the drug is difficult to tolerate, dose adjustment is recommended at the discretion of the physician.
In what cases is it prescribed
Paroxetine is taken in the following cases:
- For depression of any type, including severe cases of the disease accompanied by anxiety. Sometimes used as a preventive measure to prevent relapses of depressive conditions.
- Prescribed to patients with obsessive-compulsive disorders , as well as their prevention.
- Suffering from panic attacks, phobia of open space and crowds of people .
- Patients with social phobia leading to anxiety disorders . The disorder is associated with a feeling of fear of getting into and being in socially significant places.
- In cases of generalized anxiety disorders . Such people experience tension, anxiety, and anxiety throughout the day.
- Patients with post-traumatic stress disorder .
- Prescribed to people suffering from nightmares, obsessive thoughts and actions .
Reviews from doctors
Experts' opinions on antidepressants:
Egorov B.E.:
Like any antidepressant, Paroxetine has a severe effect on the human body. This drug is allowed to be taken only if there are really severe psychological problems. However, many patients insist on these pills even under mild stress conditions. This is strictly contraindicated.
The therapeutic effect is high, but positive results can only be achieved with strict adherence to the treatment regimen. Increasing the dose of an antidepressant on your own is life-threatening, especially for patients with suicidal tendencies.
Kalinina T.B.:
Paroxetine is a new generation antidepressant with high anxiolytic, sedative, and stimulating effects. Many of my colleagues trust older antidepressants, such as Fluoxetine. However, modern Paroxetine has a number of advantages, for example, easier adaptation of patients to the powerful antipsychotic effect of the tablets.
Neurologists insist that antidepressants are not the most accessible and simple way to get rid of depression, but a difficult therapeutic agent that requires constant medical supervision.
Self-medication is strictly unacceptable!
Criticism
Main article: Selective serotonin reuptake inhibitors § Criticism
In 2006, GlaxoSmithKline, the company that makes the drug, reported that taking paroxetine was associated with an increased risk of suicide. Several dozen lawsuits were brought against the company by victims and their family members. Lawyers for the injured parties managed to gain access to the company’s internal documentation and, based on its study, concluded that GlaxoSmithKline back in 1989 had information about an eightfold increase in the risk of suicide when taking its drugs[40]; however, the company kept this data silent for a long time[41].
The 1,374 letters from viewers (mostly patients) received by the BBC following its 2002 program on paroxetine involved acts of violence or self-harm occurring at the start of treatment with the drug or immediately after increasing its dosage. As noted by D. Healy, A. Herxheimer, DB Menkes (2006), these data cannot be considered just individual reports, since the analysis clearly indicates the connection of these actions with dosage; in addition, self-reports of acts of violence were provided by patients who were not previously prone to violent acts.[42]
According to some reports, paroxetine withdrawal symptoms can be so persistent that in these cases patients have to reduce their dosage very slowly. It was found that, according to the results of GlaxoSmithKline's own studies, withdrawal symptoms occurred in the majority of healthy volunteers taking paroxetine[43]. According to WHO, patients taking paroxetine have the most severe withdrawal problems compared to patients taking other antidepressants. GlaxoSmithKline for a long time denied the problem of addiction to paroxetine. In 2002, the FDA issued a warning and the International Federation of Pharmaceutical Manufacturers' Associations announced on US television that GlaxoSmithKline was guilty of misleading the public about paroxetine. In 2003, Glaxo, in fine print, rewrote previous estimates of the risk of withdrawal reactions in the labels from 0.2% to 25%[41].
A systematic review of 29 published and 11 unpublished clinical studies (reviewed by C. Barbui, T. Furukawa, A. Cipriani, 2008) found that paroxetine was not superior to placebo in terms of overall treatment efficacy and tolerability. These results were not biased by selective selection of published studies.[25]
Another review, conducted by the famous American psychologist Irving Kirsch and including data from 42 clinical trials of 6 antidepressants, including paroxetine (some of these studies were previously unpublished and were ignored), showed that the difference between drugs and placebo on average was only 1 .8 points on the Hamilton scale - the difference, although statistically significant, has no clinical significance.[44] According to another study conducted by Kirsch et al (a meta-analysis of 35 clinical trials of 4 antidepressants, including paroxetine), the difference between antidepressants and placebo reached clinical significance only in very severe depression [45]. The results of Kirsch's research caused widespread resonance and were discussed both in scientific journals and in the popular media[46].
Data regarding the effectiveness of paroxetine in children and adolescents are also questionable. Thus, in 2001, GlaxoSmithKline published the results of Study 329, which purported to show that Paxil (Seroxat) was an effective drug when used in this age group. The study became widely known and cited, having been cited no less than 184 times by 2010. GlaxoSmithKline even told its own sales representatives that the 329 trial "showed remarkable efficacy." In fact, the company’s internal documents admitted that the drug’s effectiveness had not been proven. In fact, Study 329 was negative for effectiveness on all eight outcomes and positive for demonstrating harm, but these facts were distorted by manipulation of the data, and the published paper of shadow authorship (with as many as 22 “authors”) ended up reporting positive effects . The New York State Attorney General sued GlaxoSmithKline in 2004 for deceiving consumers about the harms caused by paroxetine, leading to the disclosure of company records as part of the settlement. In addition to the findings that paroxetine was ineffective, it was found that at least eight children became suicidal while taking the drug, compared with only one in the placebo group. However, in an article published on the results of Study 329, five cases of suicidal ideation and behavior were defined as “emotional lability”, and three additional cases of suicidal ideation and self-harm were called “hospitalization”[41].
Four of GlaxoSmithKline's five studies of paroxetine in children and adolescents, which did not show paroxetine to be effective and demonstrated a possible increased risk of suicidal ideation and behavior, were not published.[47] Due to the suppression of data on paroxetine, which showed that the drug caused severe side effects in children, an unprecedented four-year investigation was launched in the UK in 2004. This investigation into the circumstances surrounding the marketing of paroxetine was the largest ever conducted by the UK Medicines and Healthcare Products Regulatory Agency in connection with a drug safety review[48].
Analogs
The most affordable analogues of Paroxetine:
- Fluoxetine. A time-tested antidepressant with a high therapeutic effect. It is addictive and is accompanied by severe adverse reactions in many patients. Requires constant medical supervision. Can increase suicidal tendencies. There are many known cases of deaths caused by taking Fluoxetine.
Cost - about 30 rubles for 20 tablets.
- Amitriptyline. A powerful antidepressant that can be prescribed to children from the age of six (unlike the original - prescription is allowed only from 18 years of age). However, it is strictly contraindicated for pregnant and lactating women.
Price – from 6 to 50 rubles for 50 tablets (depending on the sales region and manufacturer).
- Phenibut. A drug belonging to the group of antipsychotics and nootropics. Effectively fights psycho-emotional problems, while having a positive effect on brain function. At the discretion of the doctor, it may be prescribed during pregnancy and lactation.
The price for 20 tablets is about 90 rubles.
To purchase any of the presented analogues, a prescription in Latin from a neurologist is required.
Paroxetine tablets are high-quality help for severe psycho-emotional problems. Requires strict adherence to the treatment regimen prescribed by the doctor; it has many contraindications and adverse reactions.
The greatest danger of Paroxetine is its ability to increase suicidal tendencies, which often causes the death of the patient.
Alternative drugs
| This section is missing references to information sources. Information must be verifiable, otherwise it may be questioned and deleted. You may edit this article to include links to authoritative sources. This mark was set on May 12, 2011 . |
The antidepressant sertraline, which also belongs to the SSRI group, has a similar but less pronounced effect with less side effects.
An alternative to paroxetine for treating anxiety may be tranquilizers such as Grandaxin. Tranquilizers do not have significant side effects and are suitable for a one-time fight against anxiety: at your own wedding, exams, public speaking. Paroxetine and sertraline are used for long-term severe anxiety disorders such as panic attacks.
For depression without the anxiety component, paroxetine is preferred over newer antidepressants such as duloxetine (Cymbalta) and milnacipran (Ixel) because they act simultaneously on serotonin and norepinephrine and cause fewer side effects.