09/23/2020 Alena Masheva Health
The thalamus is a brain structure that is formed from the diencephalon during intrauterine development, making up the bulk of its mass in an adult. It is through this formation that all information from the periphery is transmitted to the cortex. The second name for the thalamus is the visual hillocks. More details about it later in the article.
Location
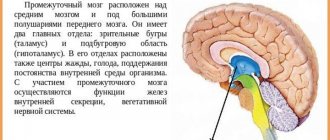
The thalamus is part of the cerebral hemispheres of the forebrain. It is located lateral to the lateral ventricles - cavities of the brain that are part of the cerebrospinal fluid (CSF) circulation system. Below it is the hypothalamus, from which the visual hillocks are separated by a groove.
Above and slightly outside the thalamus are the basal ganglia. These formations are necessary for precise, coordinated movements. These structures are separated from each other by the internal capsule - a bundle of white matter of the forebrain, through which pathways pass from the periphery to the center.
The right and left parts of the thalamus are connected to each other by interthalamic gray matter. It is present in 70% of people.
Classification of thalamic nuclei
In total, there are about 120 nuclei in the visual thalamus of the brain. Depending on their location, they are divided into three groups:
- medial;
- lateral;
- front
- specific;
- associative;
- nonspecific.
In the lateral group of nuclei, the medial and lateral geniculate bodies, as well as the cushion, are distinguished.
There is also a classification depending on the function performed by the kernels:
Specific kernels
The specific nuclei of the thalamus have a number of distinctive features. All formations of this group receive sensory information from second neurons (nerve cells) of sensitive pathways. The second neuron, in turn, can be located in the spinal cord or in one of the structures of the brain stem: medulla oblongata, pons, midbrain.
Each of the signals coming from below is processed in the thalamus and then goes to the corresponding area of the cortex. Which area the nerve impulse goes to depends on what information it carries. Thus, information about sounds goes to the auditory cortex, about objects seen - to the visual cortex, and so on.
In addition to impulses from the second neurons of the pathways, specific nuclei are responsible for the perception of information coming from the cortex, reticular formation, and brain stem nuclei.
The nuclei, which are located in the anterior part of the thalamus, ensure the conduction of impulses from the limbic cortex of the brain through the hippocampus and hypothalamus. After processing the information, it again enters the limbic cortex. Thus, the nerve impulse circulates in a certain circle.
Thalamus
Definition of the concept
The thalamus (thalamus opticus - visual thalamus) is a section of the diencephalon that controls the flow of sensory stimulation coming to it from all senses. Its main functions: transformation of sensory excitation, afferent interaction with the cortex, limbic system, strio-pallidal system, hypothalamus, and ensuring attention.
"Memory"
"Thalamic tubercle - selection for sensations." The thalamus is like a personal secretary who receives all the information, but conveys to his boss only the most important and in a concise and understandable form, and then transmits the boss’s orders to the executors.
The thalamus (“visual thalamus”) prepares sensory stimulation coming from the sense organs for transmission to certain areas of the cerebral cortex. The thalamus filters information coming from all receptors, pre-processes it and then sends it to the appropriate areas of the cortex. In addition, the thalamus communicates between the cortex, on the one hand, and the cerebellum and basal ganglia on the other. In other words, through the thalamus, lower nerve centers report to higher ones, and higher cortical nerve centers control the work of lower nerve centers.
Structure of the thalamus
The thalamus belongs to the diencephalon, which is located between the midbrain and the cerebral hemispheres of the forebrain. It consists of 40 cores. The thalamus can be said to occupy the center of the brain, consistent with its central role in processing information received by the brain.
The thalamus collects sensory excitation coming along afferent pathways from external exteroceptors and internal interreceptors and prepares it for transmission to the cortex, and then transmits it to different zones of the cortex along different afferent pathways: specific, nonspecific and associative. Only olfactory sensory stimulation comes to the thalamus from the olfactory cortex; all other sensory streams first enter the thalamus, and then from it to the cortex.
If the thalamus is damaged, the cortex may lose some sensory information and sensory perception will be impaired.
The nuclei of the thalamus are divided into specific and nonspecific. Accordingly, the paths from them to the cerebral cortex are divided into specific and nonspecific. Specific nuclei, in turn, are divided into switching and associative. Characteristics of nuclei. Specific. They are divided into switching and associative. Switchable. The flow of sensory excitation is switched from the lower nerve centers of the spinal cord and trunk to the sensory zones of the cortex. The received sensory stimulation is first recoded and processed. Ventral anterior. Regulation of movements. Ventral posterior. Switches somatosensory afferent information: tactile, proprioceptive, gustatory, visceral, partially temperature, pain. Lateral geniculate body. Switching of visual information to the occipital region of the cortex. Medial geniculate body. Switching of auditory information to the temporal cortex of the posterior part of the Sylvian fissure (Heschl's gyrus). Associative. They receive afferent signals from the switching nuclei and send them to the associative zones of the cortex. The main function is the integration of the activity of the thalamic nuclei and associative zones of the cortex, since these zones send signals to the associative nuclei. Nonspecific nuclei. Afferent signals are received from other nuclei of the thalamus along collaterals of all sensory pathways: from the motor centers of the brain stem, cerebellar nuclei, basal ganglia, hippocampus, and from the frontal lobes. Efferent outputs are to other nuclei of the thalamus, the cerebral cortex, and to other brain structures. They have a modulating effect on the cortex, activating it and providing attention.
Associative kernels
The association nuclei are located closer to the posteromedial part of the thalamus, as well as in the cushion area. The peculiarity of these structures is that they do not participate in the perception of information that comes from the underlying formations of the central nervous system. These nuclei are necessary to receive already processed signals in other nuclei of the thalamus or in overlying brain structures.
The essence of the “associativity” of these nuclei is that any signals are suitable for them, and the neurons are able to adequately perceive them. Signals from these structures enter the correspondingly named areas of the cortex—associative zones. They are located in the temporal, frontal and parietal parts of the cortex. Thanks to the receipt of these signals, a person is able to:
Pathology
Focal lesions of T. can develop in various pathol. processes, but most often - in cases of cerebral circulation disorders (ischemia, hemorrhage due to atherosclerosis and hypertension). The most common circulatory disorders are in the area of the deep branches of the posterior cerebral artery. During a stroke in this area, the ventrolateral nuclei of T are mainly damaged.
or its dorsal medial nucleus. In T., both ischemic infarctions and hemorrhages are possible due to arterial ruptures, vasculitis, hypertensive crises with high blood pressure, and arteriovenous aneurysms. Much less often, thalamic complexes are damaged in infectious, tumor, dystrophic and other diseases.
Inflammatory lesions of T. occasionally occur in tuberculosis, syphilis, sepsis, and encephalitis (viral, bacterial). Degenerative processes in the structures of T. occur due to genetic metabolic defects or intoxications (exogenous, endogenous), as well as in the residual stage of infectious or traumatic brain damage.
Wedge, the symptoms of T.'s damage are diverse and depend on the functional role of the damaged structures. Thalamic syndrome was first described in detail by J. Dejerine and G. Roussy in 1906 when T. softened due to blockage of the posterior cerebral artery and its branches. When turning off a. thalamoge-niculata on the side opposite to the lesion in T.
, the following symptoms develop: 1) hemihypesthesia or hemianesthesia with a pronounced disturbance of deep sensitivity, more on the extremities, sometimes without a disturbance of sensitivity on the face; 2) hyperpathia or dysesthesia (see Sensitivity, disorders), paroxysmal or constant severe pain spreading to the entire half of the body (thalamic pain syndrome);
3) loss of vibration sensitivity; 4) transient hemiparesis without severe muscle spasticity and patol. Babinski reflex; 5) atrophy of the muscles of the affected half of the body; 6) choreic and athetoid movements in the fingers; 7) hemiataxia; sometimes homonymous hemianopsia; 9) Nothnagel facial paresis; 10) attention disorders.
With partial damage to T., certain groups of these symptoms may predominate. When the medial part of the T. (basin a. tha-lamoperforata) is destroyed, the dentato-rubrothalamic pathway is damaged, along which impulses from the cerebellum enter the T., and hyperkinesis (athetoid, choreic) and hemiataxia occurs on the side opposite to the lesion.
When the anterior sections of T. are affected, contralateral facial paresis of the facial muscles is detected. With diffuse damage to T., thalamic syndrome is often combined with autonomic disorders (cyanosis, atrophy of the skin and nails, pastiness, coldness of the skin on the affected side of the body), more pronounced in the hand.
In this case, the hand acquires a characteristic position: the forearm is bent and pronated, the hand is bent at the wrist joint, the proximal phalanges of the fingers are bent, while the middle and distal phalanges are extended (the so-called thalamic hand). Sometimes Bernard-Horner syndrome occurs on the side of the lesion (see Bernard-Horner syndrome).
Diagnosis of thalamic syndrome is based on identifying the complex of its constituent symptoms. Wedge, diagnostic methods consist of determining superficial and deep sensitivity, determining visual fields (see), muscle strength of the limbs, coordination of movements (see), etc. Diagnosis is helped by Förster's symptom: irritation of various receptors (visual, auditory, taste) causes pain and an unpleasant sensation in the affected half of the body.
The differential diagnosis of thalamic Dejerine-Roussy syndrome should be carried out with Guesde-Holmes syndrome - unilateral affective dysesthesia, which is characterized by unilateral dysesthesia, exaggerated external manifestations of affective reactions (grimacing), sudden defensive movements in response to minor physical irritation (eg.
, slight pin prick), unilateral disorder of taste and smell. This symptom complex develops when corticothalamic and cortical-hypothalamic connections are disrupted. In addition, thalamic syndrome should be differentiated from the syndrome of damage to the internal capsule, in which, along with hemianesthesia (sometimes in combination with hemianopsia), spastic hemiplegia is observed. T.'s lesion can be detected by cerebral angiography (see), computed tomography (see Computed tomography).
Treatment of thalamic syndrome is pathogenetic. Significant difficulties arise with thalamic hemialgia, in which conventional analgesics are not very effective. It is recommended to use seduxen, aminazine, finlepsin, tegretol, etc. For hyperkinesis and intractable pain syndromes, stereotactic operations on the T. nuclei are used (see Stereotactic neurosurgery).
The prognosis of thalamic syndrome depends on the nature of the underlying pathol. process.
Bibliography: Adrianov O. S. On the principles of organization of integrative activity of the brain, M., 1976; Batuev A. S. Higher integrative systems of the brain, D., 1981, bibliogr.; GuselnikovV. I. Electrophysiology of the brain, M., 1976; Durinyan R. A. Central structure of afferent systems, L., 1965; aka, Cortical control of nonspecific brain systems, M., 1975; Kesarev V. S.
Quantitative architectonics of the human brain, Vestn. Academy of Medical Sciences of the USSR, No. 12, p. 29, 1978; Clinical neurophysiology, ed. N. P. Bekhtereva, p. 49, L., 1972; Krol M. B. and Fedorova E. A. Basic neuropathological syndromes, M., 1966; Kurepina M. M. Animal brain, M., 1981; General and particular physiology of the nervous system, ed. P. G. Kostyuk, p. 313, L., 1969; Serkov F.N. and Kazakov V.N.
Neurophysiology of the thalamus, Kyiv, 1980, bibliogr.; Vascular diseases of the nervous system, ed. E. V. Schmidt, p. 56, 330, M., 1975; Experimental Psychology, ed. S. S. Stevens, trans. from English, p. 174, M., 1960; In arraqu e r-B o g- das L. a. O. Thalamic hemorrhage, Stroke, v. 12, p. 524, 1981; Brow n J.W.
Thalamic mechanisms in language, Handb. behavioral neurobiol., ed. by F.A. King, v. 2, p. 215, N. Y, -L., 1979; DantzerR. etDela-c about ur J. Modification d'un phenomene de suppression conditionnee par une leson thalamique, Physiol. Behav., t. 8, p. 997, 1972; Dejerine J. a. Roussy G. Le syndrome thalamique, Rev. neurol. (Paris), t. 14, p. 521, 1906;
Einfiihrung in die stereotaktischen Operationen mit einem Atlas des menschlichen Gehirns, hrsg. v. G. Schaltenbrand a. P. Bailey, Bd 1, S. 230, 1959, Bibliogr.; Graeber RC a. Ebbesson SO Retinal projections in the lemon shark (Negaprion previrostris), Brain Behav. Evolut., v. 5, p. 461, 1972; G raybie 1 AM
Some fiber pathways related to the posterior thalamic region in the cat, ibid., v. 6, p. 363, 1972; Henderson VW, Alexander MP a. N aese g MA Right thalamic injury, impaired visuospatial perception, and alexia, Neurology, v. 32, p. 235, 1982, bibliogr.; K wak R., K ad o y a S. a. Suzuki T.
Factors affecting the prognosis in thalamic hemorrhage, Stroke, v. 14, p. 493, 1983; Lapresle J. a. Ha-g and en a and M. Anatomico-chemical correlation in focal thalamic lesions, Z. Neurol., v. 205, p. 29, 1973; Lejeune H. Lesions thalamiques medianes et effets differentiels dans les apprentissages operants, Physiol, a. Behav., t. 18, p. 349, 1977;
Van B ur en J. M. a. In orke RC Variations and connections of the human thalamus, pt 1 - 2, NY ao, 1972; V e 1 as with o F. a. Velasco M. A reticulothalamic system mediating proprioceptive attention and tremor in man, Neurosurgery, v. 4, p. 30, 1979; Villablanca J. a. Sali-nas-Zeballos M. E. Sleep-wakeful-ness, EEG and behavioral studies of chronic cats without the thalamus, Arch. ital. Biol., v. 110, p. 383, 1972.
D. K. Bogorodinsky, A. A. Skoromets; O. S. Adrianov (physics), V. S. Kesarev (an., comparative anatomy).
Nonspecific nuclei
This group of nuclei is called nonspecific because it receives information from almost all structures of the central nervous system:
The impulse from nonspecific nuclei also goes to all areas of the cerebral cortex. Such selectivity, as in the case of associative and specific nuclei, is absent here.
Since it is this group of nuclei that has the largest number of connections, it is believed that thanks to it, the harmonious, coordinated work of all parts of the brain is ensured.
Separately about the visual thalamus
Previously, it was believed that the thalamus processed only visual impulses, and then the organ received the name - visual thalamus. Now this name is considered obsolete, since the organ processes almost the entire range of afferent systems (except for smell).
The system that provides visual perception is one of the most interesting. The main external organ of vision is the eye, a receptor that has a retina and is equipped with special cells (cones, rods) that transform the light beam and electrical signal. The electrical signal, in turn, passing through the nerve cells enters the lateral center of the thalamus, which sends the processed signal to the central part of the cerebral cortex. Here the final analysis of the signal occurs, thanks to which what is seen is formed, that is, the picture.
Metathalamus
A separate group of nuclei of the visual thalamus is distinguished called the metathalamus. This structure consists of the medial and lateral geniculate bodies.
The medial geniculate body receives hearing information. From the underlying parts of the brain, information arrives through the upper humps of the midbrain, and from above the structure receives impulses from the auditory cortex.
The lateral geniculate body belongs to the visual system. Sensitive information to the nuclei of this group comes from the retina through the optic nerves and optic tract. The information processed in the thalamus then goes to the occipital cortex, where the primary center of vision is located.
Functions of the thalamus
How is sensitive information coming from the periphery processed and then transmitted to the forebrain cortex? This is the main role of the visual thalamus.
Thanks to this function, in case of damage to the cortex, it is possible to restore sensitivity through the thalamus. Thus, reparation of pain, temperature, as well as rough touch is possible.
Another important function of the thalamus is the coordination of movement and sensitivity, that is, sensory and motor information. This is due to the fact that not only sensory impulses enter the thalamus. It also receives impulses from the cerebellum, ganglia of the extrapyramidal system, and cerebral cortex. And these structures, as is known, take part in the implementation of movements.
The visual thalamus is also involved in maintaining conscious activity, regulating sleep and wakefulness. This function is carried out due to the presence of connections with the locus coeruleus of the brain stem and the hypothalamus.
Thalamus as a putative biomarker of mental disorders
Features of thalamic morphology in various neuropsychiatric disorders can provide important information about the onset, progression and outcome of the disorder, as well as response to treatment. The maturation of the thalamus and cortex are closely linked, such that a thalamic abnormality in early neurodevelopment can impair normal cortical development (and vice versa), and, not surprisingly, pathology of the thalamus has been implicated in the neurobiology of schizophrenia. The thalamus (from the Greek word thalamos, used to refer to the innermost room, the storeroom) can be divided into dorsal and ventral divisions: the dorsal thalamus consists of nuclei that have reciprocal connections with the cerebral cortex and striatum, while the ventral thalamus does not usually send its projections into the cortex. In humans, the dorsal thalamus is a paired structure with a strategic central location between other subcortical structures and the cortex. Two separate dorsal thalami are located at the base of each cerebral hemisphere on either side of the third ventricle, respectively. The dorsal thalamus has anterior, medial, and lateral divisions defined by a curved sheet of myelinated fibers called the internal medullary lamina. These divisions are composed of different nuclei of the thalamus, and their division is based on cytoarchitecture, connectivity patterns and functionality, with these nuclei having many functions.
There are two types of neurons in the dorsal thalamic nuclei that can be distinguished by their morphology and chemoarchitecture: locally acting gamkergic interneurons and glutamatergic relay cells projecting beyond the thalamus. The physiological properties of thalamic relay nylons indicate that they may be subject to two main types of response modes that will determine the nature of the message conveyed to the cortex: the impulse response mode is thought to be used for signal detection, whereas the tonic response mode is thought to be used by the thalamus relay cells for more accurate signal analysis. It has been suggested that disruption of modulation processes (i.e., the transition from signal analysis to signal detection) may lead to aberrant saliency, such as that observed in schizophrenia.
The dorsal thalamic nuclei are thought to receive two types of afferent fibers that are classified as modulators, regardless of their origin (i.e., cortical or subcortical), and which can be identified based on their synaptic morphology and postsynaptic actions. In this sense, drivers are essentially those afferents to the thalamus that carry the message conveyed by the thalamocortical cells, whereas modulators are essentially the afferents to the thalamus that influence how that message is conveyed by those thalamocortical cells. Thalamic nuclei can be classified according to the afferents they receive. The primary (or primary) nuclei receive their drivers from a peripheral or subcortical structure and receive their modulators from pyramidal cells in layer VI of the ipsilateral cortex. In this circuit, visual, somatosensory, and auditory afferents send peripheral sensory information to the first-order nuclei of the thalamus. On the other hand, higher order nuclei (or associations) receive their drivers from pyramidal cells located in layer V of the ipsilateral cortex. In this scheme, higher order relays transmit messages from one cortical area to another, for example, regarding output to motor or premotor centers from a cortical area. Just as sensory information passes through first-order nuclei on the way to the cortex, so cortico-cortical information passes through higher-order nuclei (with direct cortico-cortical pathways providing some other function, for example, modulatory); which challenges the traditional view of cortico-cortical signaling. With respect to the response mode of relay cells in higher order nuclei, burst and tonic modes are thought to be used in a similar way: burst mode can be used to indicate a shift in output pattern in transtochostic cortico-cortical connectivity (from one cortical area to another ), and the tonic mode is then turned on by higher order relay cells to more reliably transmit information to another area of the cortex. It should be noted that research shows that higher order nuclei have more discontinuities than first order relays. Although limited, available evidence suggests that drivers for higher-order relays originate from several different cortical regions, suggesting that relays are likely to represent several different functions. At least half of the thalamus is involved in transtochastic cortico-cortical communication, implying that disruption of this type of signaling may affect higher cortical functions. The function of the thalamo - cortical inputs to the cortex (both in the first and higher order) may be to update the cortex in the latter upon motor commands.
Traditional imaging has depicted many of the afferent and efferent connections of the thalamus, including afferent connections for first-order nuclei, as well as topographically organized first- and higher-order thalamo-cortical and cortico-thalamic pathways. Trans-synaptic neurodegeneration has been noted as a mechanism of neurodegenerative disease, in which deafferentation from cortical neurons can lead to synaptic dysfunction and hence the spread of neuronal pathology throughout vulnerable neural networks. This may be one of the mechanisms that leads to subcortical pathology, such as neuronal loss in neurodegenerative diseases, as evidenced by striatal lesions in Huntington's disease (HD), frontotemporal dementia (FTD), and Alzheimer's disease (AD). Given the topographical connectivity of the thalamus to the cortex, one would predict that the thalamus could serve as a map of structural changes in cortical afferent pathways in various neurodegenerative diseases.
From a neural circuit perspective, there are a number of well-defined anatomical loops associated with the thalamus. For example, the frontal-striatal thalamo-cortical loop lines are thought to form the core network through which motor activity and behavior are mediated and may explain the similarities in behavioral changes between frontal cortical and subcortical disorders. The thalamus is a critical center in these networks. It is hypothesized that cortico-subcortical lesions in neurodegenerative disease processes may uncouple these circuits and this underlies manifest neuropsychiatric dysfunction such as the cognitive and behavioral impairments seen, for example, in FTLD. Likewise, dysfunction of the cerebellar locular pathways is also associated with neuropsychiatric disease. Disruption of neural circuits connecting the cortex, thalamus, and cerebellum may play a significant role in the pathogenesis of schizophrenia, suggesting that disruption of this circuit may be dependent on emotional and cognitive dysfunction.
With the exception of most olfactory inputs, all sensory tracts on their way to the cerebral cortex first pass through the dorsal thalamus. The function of the dorsal thalamus was thought not to be limited to transmitting information, and indeed it has been described as a "gateway" to the cortex. In terms of the current conceptualization of thalamic function, the thalamic relay is thought to function as an interlocking gate (rather than a multi-message integrator) with its component relay cells transmitting the message from their afferent movement (whether cortical or subcortical) to the cortex; signaling occurs in either pulsed or tonic modes, although with slight variations. It's not entirely clear what benefit persists when information is sent through the thalamus before it reaches the cortex. In conceptualizing the role of the thalamus in neuropsychiatric disorders, it is useful to consider failure of neural circuits associated with the thalamus rather than neuropsychiatric symptoms arising directly from the thalamus itself. The role of thalamic pathology in the disease state might then be considered a failure of its relay functions or a gating abnormality that acts on the messages it receives.
In schizophrenia, there is a volumetric deficit in the thalamus relative to brain size, and thalamic size has been shown to negatively correlate with the severity of schizophrenia symptoms. Thalamic volume deficits have been reported in first-episode psychosis, in patients poorly medicated for schizophrenia, and in those patients taking antipsychotic drugs, and there is conflicting evidence about whether thalamic volume increases with antipsychotic treatment.