| Acetylcholine | |
| Are common | |
| Systematic name | N,N,N-trimethyl-2-aminoethanol acetate |
| Abbreviations | ACh |
| Chem. formula | СH3CO2CH2CH2N(СH3)3 |
| Physical properties | |
| Molar mass | 146.21 g/mol |
| Classification | |
| Reg. CAS number | 51-84-3 |
| PubChem | 187 |
| Reg. EINECS number | 200-128-9 |
| SMILES | O=C(OCC[N+](C)(C)C)C |
| InChI | 1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 OIPILFWXSMYKGL-UHFFFAOYSA-N |
| Codex Alimentarius | E1001(i) |
| ChEBI | 15355 and 40559 |
| ChemSpider | 182 |
| Data given is based on standard conditions (25 °C, 100 kPa) unless otherwise stated. | |
Acetylcholine
(lat. Acetylcholinum), abbr.
ACH
is an organic compound, a quaternary ammonium base, a choline derivative, a neurotransmitter involved in neuromuscular transmission, and also the main neurotransmitter in the parasympathetic nervous system. In the body it is very quickly destroyed by a specialized enzyme - acetylcholinesterase.
Properties
It is used as a medicinal substance and for pharmacological studies. This compound is obtained synthetically in the form of chloride or other salt.
Chemical
Acetylcholine is an acetyl derivative of the quaternary ammonium compound choline. It is formed in the nerve cells of humans and animals from choline as a result of an enzymatic reaction [1], under the action of choline acetyltransferase, the acetyl group (CH3CO-) is transferred from acetyl-CoA to the choline substrate molecule, with the formation of coenzyme A and ACH according to the reaction equation:
acetyl-CoA + choline ⇌ {\displaystyle \rightleftharpoons } acetylcholine + CoA-SH.
The presence of this enzyme in nerve cells classifies such a cell as “ cholinergic”
» neuron.
This is a chemically unstable substance, which in the body, with the participation of a specific enzyme cholinesterase (acetylcholinesterase), is easily destroyed to form choline and acetic acid.
Physical
Colorless crystals or white crystalline mass. Dissolves in the air. Easily soluble in water and alcohol. When boiled and stored for a long time, the solutions decompose.
Medical
The physiological cholinomimetic effect of acetylcholine is due to its stimulation of the terminal membranes of M- and N-cholinergic receptors.
The peripheral muscarinic-like effect of acetylcholine manifests itself in a slowdown of heart contractions, expansion of peripheral blood vessels and a decrease in blood pressure, increased peristalsis of the stomach and intestines, contraction of the muscles of the bronchi, uterus, gall and bladder, increased secretion of the digestive, bronchial, sweat and lacrimal glands, miosis. The miotic effect is associated with increased contraction of the orbicularis iris muscle, which is innervated by postganglionic cholinergic fibers of the oculomotor nerve. At the same time, as a result of contraction of the ciliary muscle and relaxation of the ligament of cinnamon of the ciliary girdle, a spasm of accommodation occurs.
Constriction of the pupil caused by the action of acetylcholine is usually accompanied by a decrease in intraocular pressure. This effect is partly explained by the fact that when the pupil narrows and the iris flattens, Schlemm's canal (venous sinus of the sclera) and fountain spaces (spaces of the iridocorneal angle) expand, which ensures better outflow of fluid from the internal media of the eye. It is possible that other mechanisms are also involved in the decrease in intraocular pressure. Due to their ability to reduce intraocular pressure, substances that act like acetylcholine (cholinomimetics, anticholinesterase drugs) are widely used for the treatment of glaucoma. It should be borne in mind that when these drugs are introduced into the conjunctival sac, they are absorbed into the blood and, having a resorptive effect, can cause side effects characteristic of these drugs. It should also be borne in mind that long-term (over a number of years) use of miotic substances can sometimes lead to the development of persistent (irreversible) miosis, the formation of posterior petechiae and other complications, and long-term use of anticholinesterase drugs as miotics can contribute to the development of cataracts.
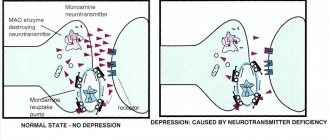
Acetylcholine also plays an important role as a CNS mediator. It is involved in the transmission of impulses in different parts of the brain, with small concentrations facilitating and large concentrations inhibiting synaptic transmission. Changes in acetylcholine metabolism lead to severe disruption of brain function. Its deficiency largely determines the clinical picture of such a dangerous neurodegenerative disease as Alzheimer's disease. Some centrally acting acetylcholine antagonists (see Amizil) are psychotropic drugs (see also Atropine). An overdose of acetylcholine antagonists can cause disturbances in higher nervous activity (have a hallucinogenic effect, etc.). The anticholinesterase effect of a number of poisons is based precisely on the ability to cause the accumulation of acetylcholine in synaptic clefts, overexcitation of cholinergic systems and more or less rapid death (chlorophos, karbofos, sarin, soman) (Burnazyan, “Toxicology for medical university students,” Kharkevich D.I., “ Pharmacology for students of the Faculty of Medicine").
Acetylcholine synthesis
It occurs in the cytoplasm in nerve endings. The reserves of the substance are located in presynaptic terminals in the form of vesicles. The occurrence of an action potential leads to the release of acetylcholine from several hundred “capsules” into the synaptic cleft. The substance released from the vesicles binds to specific molecules on the postsynaptic membrane. This increases its permeability to sodium, calcium and potassium ions. The result is an excitatory postsynaptic potential. The effect of acetylcholine is limited through its hydrolysis with the participation of the enzyme acetylcholiesterase.
Application
General Application
Acetylcholine chloride (lat. Acetylcholini chloridum)
). Acetylcholine chloride is not widely used as a medicine.
Treatment
When taken orally, acetylcholine is hydrolyzed very quickly and is not absorbed from the mucous membranes of the gastrointestinal tract. When administered parenterally, it has a quick, sharp and short-lasting effect (like adrenaline). Like other quaternary compounds, acetylcholine penetrates poorly from the vascular bed through the blood-brain barrier and does not have a significant effect on the central nervous system when administered intravenously. Sometimes in experiments, acetylcholine is used as a vasodilator for spasms of peripheral vessels (endarteritis, intermittent claudication, trophic disorders in the stumps, etc.), and for spasms of the retinal arteries. In rare cases, acetylcholine was administered for intestinal and bladder atony. Acetylcholine has also sometimes been used to facilitate x-ray diagnosis of esophageal achalasia.
Form of application
Since the 1980s, acetylcholine has not been used as a medicine in practical medicine (M. D. Mashkovsky, “Medicines,” volume 1), since there are a large number of synthetic cholinomimetics with a longer and more targeted effect. It was prescribed subcutaneously and intramuscularly at a dose (for adults) of 0.05 g or 0.1 g. Injections, if necessary, were repeated 2-3 times a day. When injecting, you had to make sure that the needle did not enter a vein. Intravenous administration of cholinomimetics is not allowed due to the possibility of a sharp decrease in blood pressure and cardiac arrest.
Danger of use during treatment
When using acetylcholine, it should be taken into account that it causes a narrowing of the coronary vessels of the heart. In case of an overdose, a sharp decrease in blood pressure with bradycardia and heart rhythm disturbances, profuse sweat, miosis, increased intestinal motility and other phenomena may be observed. In these cases, 1 ml of a 0.1% solution of atropine (repeatedly if necessary) or another anticholinergic drug should be immediately injected into a vein or under the skin (see Metacin).
Features of muscarinic molecules
M-cholinergic receptors belong to the serpentine class. They transmit impulses through heterotrimeric G proteins. A group of muscarinic receptors were identified due to their ability to bind the alkaloid muscarine. Indirectly, these molecules were described at the beginning of the 20th century while studying the effects of curare. Direct research of this group began in the 20-30s. the same century after identifying the compound acetylcholine as a neurotransmitter that supplies impulses to neuromuscular synapses. M-proteins are activated by muscarine and blocked by atropine, n-molecules are activated by nicotine and blocked by curare.
Over time, a large number of subtypes were identified in both groups of receptors. Only nicotinic molecules are present at neuromuscular synapses. Muscarinic receptors are found in glandular and muscle cells, and also, together with n-cholinergic receptors, in neurons of the central nervous system and nerve ganglia.
Participation in life processes
The (endogenous) acetylcholine formed in the body plays an important role in vital processes: it takes part in the transmission of nervous excitation in the central nervous system, autonomic nodes, and the endings of parasympathetic and motor nerves. Acetylcholine is associated with memory functions. A decrease in acetylcholine in Alzheimer's disease leads to memory impairment in patients. Acetylcholine plays an important role in falling asleep and waking up. Arousal occurs with increased activity of cholinergic neurons in the basal ganglia of the forebrain and brain stem.[2]
Acetylcholine is produced by neurons in the basal ganglia, which are activated during concentration.[3][4]
Physiological properties
Acetylcholine is a chemical transmitter (mediator) of nervous excitation; the endings of the nerve fibers for which it serves as a mediator are called cholinergic, and the receptors that interact with it are called cholinergic receptors. The cholinergic receptor (according to modern foreign terminology - “cholinergic receptor”) is a complex protein macromolecule (nucleoprotein) localized on the outer side of the postsynaptic membrane. In this case, the cholinergic receptors of postganglionic cholinergic nerves (heart, smooth muscles, glands) are designated as m-cholinergic receptors (muscarinic-sensitive), and those located in the area of ganglionic synapses and in somatic neuromuscular synapses are designated as n-cholinergic receptors (nicotine-sensitive). This division is associated with the characteristics of the reactions that occur during the interaction of acetylcholine with these biochemical systems: muscarinic-like in the first case and nicotine-like in the second; m- and n-cholinergic receptors are also located in different parts of the central nervous system.
According to modern data, muscarine-sensitive receptors are divided into M1-, M2- and M3-receptors, which are distributed differently in organs and are heterogeneous in physiological significance (see Atropine, Pirenzepine).
Acetylcholine does not have a strict selective effect on the types of cholinergic receptors. To one degree or another, it acts on m- and n-cholinergic receptors and on subgroups of m-cholinergic receptors. The peripheral nicotine-like effect of acetylcholine is associated with its participation in the transmission of nerve impulses from preganglionic fibers to postganglionic fibers in the autonomic ganglia, as well as from motor nerves to striated muscles. In small doses it is a physiological transmitter of nervous excitation; in large doses it can cause persistent depolarization in the area of synapses and block the transmission of excitation.
Explanations
The above classification is determined by the specificity of the reactions that occur when these biochemical systems and acetylcholine interact. This, in turn, explains the reasons for some processes. For example, a decrease in pressure, increased secretion of gastric, salivary and other glands, bradycardia, constriction of the pupils, etc. when affecting muscarine-sensitive proteins and contraction of skeletal muscles, etc. when affecting nicotine-sensitive molecules. Moreover, recently scientists have begun to divide m-cholinergic receptors into subgroups. The role and localization of m1- and m2-molecules are the most studied today.
Sources
- Acetylcholine: general information. Human Biology Knowledge Base
. - Rockland, K. S. Brain. In A. E. Kazdin (Ed.), Encyclopedia of psychology (Vol. 1, pp. 447–455). Washington, DC: American Psychological Association.
- How attention helps you remember Anne Trafton, MIT News Office September 27, 2012
- Memory formation linked to auxiliary brain cells
| Neurotransmitters | |
| |