Antidepressants are one of the most difficult groups of drugs for pharmaceutical consultation. Often associated with very specific disorders and sold strictly by prescription, they are shrouded in many secrets and speculation. Some patients are afraid of antidepressants, others have too high hopes for them. But both the former and the latter have many questions about how these medications will affect their lives. They ask these questions not only to the attending physician, but also to another person in a white coat - the head of the pharmacy. We have collected answers to the most popular of them in this material to help our readers, if necessary, build a capacious and correct consultation.
Explain in simple terms - how do antidepressants work?
Antidepressants are a group of psychotropic medications that affect the levels of neurotransmitters (they transmit nerve impulses between cells in the human brain), thereby helping to treat depression. We are talking about three hormones: serotonin, dopamine and norepinephrine. According to the prevailing point of view in scientific circles, with depression their concentration noticeably decreases, which is why the interaction between neurons is disrupted. Modern antidepressants help reverse this process, returning the brain to normal functioning. Thanks to this, patients experience the following improvements: improved mood, improved vitality, decreased anxiety and apathy, and normalized sleep and appetite.
Affective disorders
They are mood disorders
. In the ICD they are numbered F30 - F39[5]. They include depression, mania and their alternations (bipolar disorder, cyclothymia, etc.). These disorders, despite the apparent differences (whoever has seen depressive and manic patients will certainly note how different everything is in them - appearance, thinking, behavior), have quite a lot in common: firstly, they lead to the patient’s mood becoming inadequate, secondly, they have a similar pharmacological nature and, accordingly, are often treated with the same drugs; thirdly, they can “flow” from one to another (for example, what was incorrectly diagnosed as a depressive episode - F32, may actually turn out to be a BAR - F31).
Therefore, we will consider them all within one section, paying attention to differences where necessary. For the purposes of this post, we will look at depression and leave other mood disorders as the topic of future articles.
Depression
Perhaps the most famous of the affective disorders. Like many other terms from psychiatry, the word " depression"
“has found quite wide use in colloquial language[6], where it has lost its terminological precision: “Oh, I’m depressed,” the young ladies sigh languidly, “yesterday he left me.”
A surprise for many will be the fact that the vast majority of cases of using this term in everyday life are incorrect. Depression is not a bad mood
, not grief, not depression and not fatigue. All of the above may be symptoms of depression, but they are not its essence[7].
The classic definition of depression assumes the presence of the so-called. " depressive triad"
"[8].
Its first component is anhedonia
- a decrease in the ability to receive pleasure: what a person previously liked ceases to please and please: neither sleep, nor food, nor sex, nor career achievements - nothing causes an emotional uplift, nothing is subjectively significant and desirable .
The second component is thinking disorders
. These are not the thinking disorders that occur in schizophrenia, but their own, specifically depressive ones. First, in cases of severe depression, there is usually a decrease in the rate of thinking. Secondly, depression is characterized by certain patterns of thinking that determine pessimistic judgments and their self-blaming nature.
The third component is motor retardation
: the patient moves heavily, slowly and reluctantly.
It should be noted that there are patients in whom not all components of the triad are clearly expressed, but who nevertheless suffer from depression in one form or another [9]. Personally, I am close to the position of Beck, who considers cognitive distortions to be the main and defining component of depression [10], and deduces all other features from them.
How is depression different from grief and other unpleasant but normal conditions? With your inadequacy. The fact is that, in itself, grief is not necessarily something pathological, and can be a completely adequate response to the events that have occurred[6]. For example, if a person’s beloved hamster, to which he was attached, died, the presence of signs of the depressive triad for several days after this event is not yet a reason to claim that he (the person) suffers from depression.
However, if years have passed since the unpleasant event, the person has long ago got a new hamster, his daughter has happily married and given birth to a grandson, the person himself has recently been promoted to his favorite job, and he has successfully completed a complex project, but all his thoughts still revolve around his deceased pet, he spends his days blaming himself and nothing gives him pleasure, and getting out of bed in the morning has become incredibly difficult, then it makes sense to send such a person to a specialist: it is quite possible that he has developed depression.
There is a good video about what depression is on TED-ED:
Now that we have defined what depression is, we can talk about its causes. In this article, we will not consider the psychological aspects of depression (more on this in future articles), but will focus on the biochemistry of the brain.
As is often the case in psychiatry, there is still no consensus in the literature about what depression is at the neurochemical level[9]. There are only a number of more or less common hypotheses and evidence in favor of each of them.
Historically, the first assumption was that depression
directly
related to a lack of
certain
neurotransmitters
, in particular serotonin and norepinephrine [7,9].
The basis for this hypothesis is quite simple and clear: drugs that affect the reuptake, release or destruction of these neurotransmitters lead to improvement in at least some depressed patients.
And drugs that reduce the amount of serotonin and norepinephrine, on the contrary, cause depressive symptoms.
The monoamine hypothesis can be visualized as the following picture, with the normal state on the left and depression on the right (the neurotransmitter is shown as triangles):
In the last article, when we talked about schizophrenia, we talked mainly about dopamine, but now we will talk about serotonin
and
norepinephrine
.
Let's start with the last one. So, in our brain we have noradrenergic neurons, i.e. those that use the neurotransmitter norepinephrine as a means of signal transmission. The main accumulation of such neurons in the brain is the so-called. bluish spot
(locus coeruleus)[11]:
It is part of the reticular formation and is located in the brain stem at the level of the pons. The axons of its neurons ascend to the upper layers of the cerebral cortex, hippocampus, amygdala, septum, striatum, and cerebellar cortex. Descending projections go to the spinal cord to sympathetic and motor neurons.
Some of these projections (neuronal processes from the locus coeruleus extending to other areas of the brain) are of great importance in the formation (and treatment) of depressive symptoms.
For example, one of the projections to the prefrontal cortex is responsible for regulating mood [9]:
Another is for the effect of norepinephrine on attention:
Projection to the limbic system affects emotions, energy, fatigue and psychomotor activity:
The projection to the cerebellum may be responsible for the regulation of motor activity and the presence/absence of tremor:
Main function of the locus coeruleus
— determine what attention will be paid to: external stimuli or internal sensations[9]. In addition, it plays an important role in cognition and mood control. And if we remember that depression is characterized by a decrease in cognitive and mnestic abilities, a deterioration in the ability to voluntarily concentrate attention, as well as, in fact, inadequate mood [8], then it will become clear why the locus coeruleus is important in the etiology and treatment of depression.
Each noradrenergic neuron has receptors (it must somehow receive signals from its fellows and control its own release of neurotransmitter). These receptors can be divided, firstly, into
and
postsynaptic
(depending on location), and, secondly, into
alpha
and
beta
subtypes, which are then divided according to mediated effects, localization, as well as affinity for various substances into α1-, α2-, β1-, β2, β3-adrenergic receptors[9].
What is important for us here is that postsynaptic
α1-, α2-, β1-receptors are the main channels for signal transmission, and
presynaptic
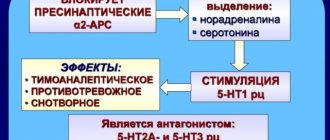
α2-receptors provide negative feedback: when there is too much norepinephrine in the synaptic cleft, it binds to these receptors, and the neuron stops releasing this neurotransmitter [12]. Accordingly (and this is important for treatment), if we block this receptor with an antagonist, we will thus “break the brake” and the amount of norepinephrine in the synaptic cleft will increase. The work of some antidepressants is based on this principle, but more on that later.
Having understood a little about noradrenergic transmission, let's move on to consider serotonergic
.
serotonin
as a messenger .
Such neurons have their own receptors, which can also be divided into pre- and postsynaptic. The first include 5HT1A and 5HT1D. The second ones, respectively, are 5HT1A (the same type of receptor can be both pre- and postsynaptic), 5HT1D, 5HT2A ( hello, psychonauts!
), 5HT2C, 5HT3, and 5HT4[9].
Presynaptic receptors, as in the case of noradrenergic neurons described above, serve the function of providing negative feedback
: when there is a lot of serotonin in the synaptic cleft, its molecule binds to the presynaptic receptor, and the neuron stops its release and, accordingly, the transmission of impulses.
Presynaptic receptors 5HT1D and 5HT1A differ in this context in that the former inhibits the release of serotonin, and the latter stops it altogether. This is interesting to us in the context that substances that are antagonists of the 5HT1D receptor can enhance serotonergic transmission.
Further - more wonderful and more wonderful. The serotonergic neuron has not only serotonergic but also noradrinergic receptors as presynaptic receptors [9]. This means that the concentration of norepinephrine affects the release of serotonin
.
As in the case of noradrinergic neurons, we are talking about α2 receptors. This means that a drug that blocks these receptors (their antagonist) will increase not only noradrenergic, but also serotonergic transmission. mirtazapine
works, for example .
But that's not all. On the surface of the body of the serotonergic neuron there are α1 receptors (also noradrenergic), which work not as a “brake”, but as an “accelerator pedal”, providing positive feedback: by connecting with such a receptor, a molecule of norepinephrine (or any other agonist) enhances the release of the neuron serotonin and, accordingly, impulse transmission [12].
Serotonergic neurons are grouped in the brain stem: in the pons and raphe nuclei. The projections of these neurons to the cortex influence mood (i.e., are directly related to the affective disorders discussed here)[9]:
Projections to the basal ganglia influence movement, as well as obsessions and compulsions (this is why SSRI antidepressants are used to treat obsessive-compulsive disorder [13]).
Projections into the limbic system are associated with anxiety and panic (5HT2A and 5HT2C receptors play a special role in this),
and projections to the hypothalamus - with the regulation of appetite and eating behavior (SSRIs are also used to treat bulimia by acting on 5HT3 receptors):
Projections to the diencephalon are responsible for insomnia (which often occurs with depression), and again the 5HT2A receptors play a major role here:
And finally, about the sad thing: projections to the spinal cord are responsible for the occurrence of erectile dysfunction (a common side effect of taking SSRIs, along with decreased libido):
So, summing up all of the above, we can conclude that serotonin deficiency
will lead to depression, anxiety, panic, can provoke phobias, obsessions, compulsions and even cause gluttony.
And finally, dopamine
. The role of dopamine and dopaminergic pathways was discussed in detail in the previous part of the article; here we will briefly limit ourselves to a description of the role of this neurotransmitter and the corresponding neurons in the etiology and treatment of depression.
Let's start with the fact that today there is a connection between depression and the presence of a deficiency in dopaminergic transmission, and also that agonists (or partial agonists) of dopamine receptors may be useful for the treatment of depressive conditions [1]. There is evidence that D2 receptor density increases in depression[2], which also suggests that dopamine agonists or dopamine reuptake inhibitors may be useful in depression. In addition, medications that increase dopaminergic transmission help with apathy and lack of energy[3], as well as abulia, which is also common in some depressed patients.
In general, the breakdown of neurotransmitters by emotion can be presented in the form of this picture [3]:
And the medications used to treat it, respectively, are in the form like this[3]:
It should be noted here that the monoamine hypothesis is currently considered somewhat “naive”, and in fact everything is much more complicated - complex processes of regulation of receptor density and gene expression mechanisms take place, but this is all beyond the scope of our article. To understand the basic functioning of antidepressants at the conceptual level, the monoamine hypothesis is quite sufficient.
What happens if during treatment the patient stops taking the prescribed drug?
It all depends on the situation. If the withdrawal is abrupt, there is a high risk that the body will not have time to adapt to the new regime, and the side effects will increase exponentially. This is called withdrawal syndrome and can be expressed in different ways. Most often, patients complain of dizziness, headaches, sleep disorders, spasms and tremors in the limbs. There is also a rebound syndrome, in which untimely withdrawal from the drug provokes the return of all the negative symptoms of depression. With gradual withdrawal, such consequences do not occur, so it is extremely important to adhere to the dosage regimen prescribed by the doctor.
Part one, in which we look with amazed children’s eyes and understand nothing at all
There have always been many questions about antidepressants. Unlike “ordinary” medicines, you can’t understand them with your mind, you just have to believe. For example, all antidepressants begin to act only in the second week of use. Traditionally, guidelines explain this by saying that “the medication accumulates during this time.” But what does “accumulate” mean? After all, the drug either acts immediately or does not act at all. All other psychotropic substances act almost immediately. Sleeping pills work immediately. Tranquilizers work immediately. Neuroleptics, mood stabilizers, anticonvulsants, stimulants - they all begin to act quickly. And “stubborn” antidepressants - only after two weeks. The question is, why?
For an explanation of some terms from the field of psychopharmacology, see the Glossary at the end of the article. - Ed.
Figure 1. Advertising for Thorazine (trade name for chlorpromazine in the United States) recommends the use of the drug for schizophrenic delusions and hallucinations.
Antidepressants improve the emotional state of patients with depression and do not affect the mood of healthy people. It is not normal. If a substance has a psychoactive effect, it has the same effect on both pathological and physiological processes. If you give a person suffering from insomnia a sleeping pill, he will fall asleep. And if you give sleeping pills to a healthy person, he will fall asleep in the same way. If an anxious and agitated patient is given a benzodiazepine tranquilizer, the person will calm down and become quiet. What about an ordinary person? The same thing - the person will calm down and calm down. The “old evil” classic neuroleptics (the same chlorpromazine - “Aminazine”) are still indispensable for acute psychoses: in fact, they have a carpet bombing effect on the rebellious brain, stopping mental activity through a break in dopamine transmission.
And if a healthy person is tied up and injected with neuroleptics, then the same thing will happen to his mental processes - the brain will turn into a scorched desert. But with antidepressants the picture is completely different. They have an effect on a depressive mood background, but not on a normal one. A healthy person will only experience side effects, but no change in consciousness. So how does the pill know which person is depressed and which isn't?
A key symptom of depression is decreased mood. This in itself is not a problem: there are a lot of substances that quickly and guaranteed elevate your mood - for example, a 40% solution of ethyl alcohol or psychostimulants, and they will take effect not after two weeks, but from the very first dose. Why then does medicine not use them? The fact is that they absolutely do not help with depression: neither alcohol nor amphetamines have an antidepressant effect, no matter what people say. And antidepressants - such as serotonin reuptake inhibitors Prozac, Zoloft, Paxil, Cipramil - help. Why?
Molecular basis of psychoses
An increase in the flow of dopamine and, to a lesser extent, serotonin is associated with the development of psychosis. According to the dopamine hypothesis of schizophrenia, the antipsychotic effect of antipsychotics is explained by their ability to block dopamine receptors. However, since there are many varieties of these receptors in the brain and they perform a wide variety of functions, it is impossible to completely silence them all, as classic antipsychotics (aminazine, haloperidol, chlorprothixene and others) do - there will be a lot of unpleasant side effects. Therefore, modern atypical antipsychotics selectively block certain types of dopamine and serotonin receptors, and they have much fewer side effects.
Symptoms of depression
The main symptom of clinical depression (or major depressive disorder) is a persistent decrease in emotional background, which cannot be reduced to simply a “bad mood”, which any person can have. Clinical depression is an illness that has clear criteria and is described by a whole bunch of symptoms. Depression manifests itself in low mood, feelings of guilt and hopelessness, loss of interests and pleasure, decreased energy, and as a result increased fatigue and decreased activity, all of which must persist for at least two weeks.
Monoamine oxidase (MAO)
This is an enzyme involved in the destruction of monoamines (more precisely, the catabolism of monoamines through oxidative deamination). Monoamines include a lot of endogenous hormones and neurotransmitters, including all those that interest us: dopamine, adrenaline, norepinephrine, serotonin and histamine; as well as exogenous psychoactive substances (for example, phenylethylamine and narcotic substances derived from it).
MAO is one of the key enzymes in the human brain, and there are about forty related proteins in the monoamine receptor family. If MAO is blocked, monoamines will stop being destroyed and will begin to accumulate in the synaptic transmission system, which is not always good. Therefore, even modern selective MAO inhibitors - very powerful antidepressants - are quite dangerous to use and are allowed only in a hospital setting, where there is constant monitoring and control over the patient’s condition.
Are antidepressants dangerous for the body?
As with any medicine, side effects may occur when taking antidepressants. Most often we are talking about drowsiness (and sometimes, on the contrary, insomnia), inhibition of reactions, hypotension. However, given that on the other side of the scale are often the patient’s thoughts about leaving life or causing other harm to himself, this is not regarded as a serious obstacle.
Some antidepressants are contraindicated for heart diseases (arrhythmia, conduction disorders in the myocardium), but there are drugs that are allowed to be taken even by people who have had a heart attack. A number of such drugs cannot be prescribed for pathologies of the kidneys and liver. Knowing the patient’s health characteristics, the doctor selects the most suitable option for him. Therefore, antidepressants can only pose a danger when self-medicated.
ANTIDEPRESSANTS in cardiological practice
Depression and cardiovascular disease are closely linked. In recent years, evidence has emerged that depression is an independent risk factor for coronary heart disease (CHD). At the same time, in some patients with coronary artery disease, depression can develop secondarily, as a personal reaction to a severe somatic illness. According to domestic and foreign studies, the prevalence of depression among patients with coronary artery disease is approximately 20%.
In patients with coronary artery disease, depression not only aggravates the clinical condition and complicates rehabilitation, but also shortens life expectancy. The results of recent studies indicate that depression increases the risk of coronary accidents and coronary death in patients with coronary artery disease. The mortality rate in patients who have had an MI and suffer from depression is 3-6 times higher than in patients who have had an MI and do not have signs of depression.
Despite its widespread prevalence, depression in cardiac patients in most cases goes unrecognized and untreated. This entails repeated visits to the cardiologist, more and more new examinations, and both the patient and his attending physician are not satisfied with the results of treatment. This situation is primarily due to the fact that patients, as a rule, do not present actual depressive complaints, such as depressed mood and loss of interests. The clinical picture is dominated by chronic pain syndrome (cardialgia), various sleep disorders, increased fatigue, decreased activity, impaired appetite, weight changes, decreased performance, problems with concentration, decreased libido, panic attacks or permanent autonomic disorders. The described symptoms are characteristic of the so-called somatized or hidden, masked depression [1].
A certain role in the underdiagnosis of depression in cardiac patients is played by the fact that cardiologists do not fully master the technique of examining depressed patients. Identification of depressive disorders is greatly facilitated by the use of psychometric scales and tests, including subjective ones (the patient answers the questions himself). Among the subjective psychometric scales for screening depression, the most famous are the Hospital Anxiety and Depression Scale (A. Zigmond, 1983) and the Beck Depression Inventory (A. Beck, 1961). Repeated use of psychometric scales makes it possible to study the dynamics of patients' condition during treatment. Considering the negative impact of depression on the prognosis, it is advisable to screen for depression among the most vulnerable categories of patients with coronary artery disease - patients with unstable angina, people who have experienced acute coronary syndromes, including myocardial infarction, as well as patients who have undergone CABG surgery.
According to the currently accepted concept, mild to moderate depression in cardiac patients can be treated by a cardiologist or general practitioner. This became possible thanks to the emergence in recent years of a number of new highly effective antidepressants that, unlike classical tricyclic antidepressants (amitriptyline, etc.), do not have pronounced behavioral toxicity and negative side effects on the cardiovascular system.
The use of tricyclic antidepressants in cardiac patients is extremely undesirable due to the somatotropic effects of these drugs, including their effect on the conduction system of the heart. The administration of these drugs in therapeutic doses is accompanied by a prolongation of the PQ, QRS and QT intervals, especially in patients with underlying conduction disorders [2]. Tricyclic antidepressants that block fast sodium channels have been shown to exhibit the properties of guanithidine-like class IA antiarrhythmic drugs, which, according to a number of studies, including the Cardiac Arrhythmia Suppression Trial (1992), increase the mortality rate in patients with coronary artery disease. Among the side effects characteristic of tricyclic antidepressants and significantly limiting their use in cardiology is reflex tachycardia. The results of the well-known Framingham study indicate that in patients with established coronary artery disease, drugs that increase heart rate (HR) should be avoided, since an increase in heart rate correlates with an increase in mortality in this category of patients. It is obvious that tricyclic antidepressants should not be prescribed to patients who are at high risk of developing coronary artery disease. Another adverse side effect of tricyclic antidepressants is orthostatic hypotension, which is most pronounced in the first two weeks of therapy and is especially characteristic of elderly patients. It is also impossible not to take into account the adverse behavioral effects that occur during treatment with tricyclic antidepressants - drowsiness, decreased level of attention, memory impairment, difficulty in intellectual activity, and impaired fine coordination of movements. In an effort to minimize the side effects described above, doctors often prescribe tricyclic antidepressants in very small doses (for example, 1/4-1/2 tablets of amitriptyline per day), which are not enough to obtain an antidepressant effect (the minimum therapeutic dose of amitriptyline is 2-3 tablets per day ). Numerous somatotropic and behavioral effects of tricyclic antidepressants are associated with their nonselectivity - the effect on several groups of CNS receptors (α1-adrenergic receptors, serotonin, muscarinic and histamine H1 receptors).
Unlike first-generation drugs, modern antidepressants are selective and therefore lack many of the side effects characteristic of tricyclic antidepressants.
Newer antidepressants include:
- selective serotonin reuptake inhibitors: citalopram (Cipramil), paroxetine (Paxil), fluoxetine (Prozac), fluvoxamine (Fevarine), sertraline (Zoloft);
- selective serotonin reuptake stimulators: tianeptine (Coaxil);
- tetracyclic antidepressants: mianserin (lerivon);
- selective serotonin and norepinephrine reuptake inhibitors: milnacipran (Ixel).
Selective antidepressants have fairly high antidepressant activity. In terms of the severity of their antidepressant action, they are somewhat inferior to classical tricyclic antidepressants, but surpass them when it comes to tolerability and safety of use. In this regard, selective antidepressants can be considered as first-line drugs in patients with cardiovascular diseases, as well as in elderly patients.
The most widely used drugs today are selective serotonin reuptake inhibitors (SSRIs). Drugs in this group inhibit the reverse penetration of serotonin from the synaptic cleft into the presynaptic neuron and do not have a significant effect on other neurotransmitters. SSRIs get their name because they are more selective in blocking serotonin reuptake than norepinephrine reuptake (at least 10-fold). In addition, SSRIs have little affinity for α1-adrenergic receptors, m-cholinergic receptors, and histamine H1 receptors, which ensures their good tolerability. SSRIs, unlike tricyclic antidepressants, do not have the ability to block slow sodium channels, and therefore they are safer in overdose.
SSRI drugs have a favorable cardiac profile. Thus, in a study by S. Roose et al. [3], who studied the safety of SSRIs in patients with coronary artery disease with concomitant depression, showed that 7-week therapy with fluoxetine at a dose of 60 mg/day did not cause any cardiac complications, did not affect blood pressure (BP), conductivity and ventricular function. ectopic activity and was accompanied by a statistically significant decrease in heart rate by 5 beats/min. It should be noted that 47% of patients included in the study had previously suffered an MI.
In patients with cardiovascular disease, the benefits of SSRIs are especially pronounced when compared with tricyclic antidepressants. A prospective randomized controlled 6-week comparative study of paroxetine (at a daily dose of up to 40 mg) and nortriptyline in patients with coronary artery disease with depression who had suffered an MI no earlier than 3 months before inclusion in the study clearly showed the benefits of SSRIs [4]. Paroxetine therapy was not accompanied by statistically significant changes in blood pressure, heart rate, heart rhythm or conduction disturbances. Due to cardiac complications, only one patient from the paroxetine group and 7 patients from the nortriptyline group dropped out of the study early (a total of 81 patients participated in the study). On the background of nortriptyline, an increase in average heart rate by 11% was noted (from 75 to 83 beats/min); there was also a statistically significant increase in cases of orthostatic hypotension and disturbances in the process of myocardial repolarization, according to ECG data. Moreover, the drugs were equally effective in relieving depressive disorders in patients with coronary artery disease.
In 1999, data from the Sertraline Antidepressant Heart Attack Trial [5] were published, the purpose of which was to study the effectiveness and safety of one of the SSRI drugs, sertraline, and its effect on the cardiac profile in patients with clinically significant depression that developed after MI. The study included patients who had a MI (5-30 days after a heart attack) with an ejection fraction of 35% or more. 16-week therapy with sertraline at a dose of 50-200 mg/day did not have a significant effect on blood pressure, heart rate, myocardial conductivity and left ventricular ejection fraction.
Although all SSRIs have a similar mechanism of action, individual drugs in this group differ in chemical structure, as well as in the degree of binding to non-serotonin receptors of the central nervous system, that is, in the degree of selectivity. Citalopram has the greatest selectivity in the SSRI group. The high selectivity of the drug ensures its good tolerability and safety in the most vulnerable groups of patients (elderly patients with cardiovascular diseases, organic lesions of the central nervous system). According to our data, the use of citalopram in a therapeutic dose of 20 mg/day in patients with coronary artery disease who have had a myocardial infarction is not accompanied by cardiotoxic effects - fluctuations in blood pressure, heart rate, disturbances in heart rhythm and conduction, including according to 24-hour ECG monitoring. A meta-analysis of several studies involving more than 1,400 patients treated with citalopram (30% were elderly) showed that citalopram does not have a significant effect on the duration of the PQ, QRS, QT intervals and myocardial repolarization processes [6]. Citalopram has a minimal risk of drug-drug interactions compared to other antidepressants and therefore is well combined with drugs that are regularly taken by patients with cardiovascular diseases (β-blockers, nitrates, calcium antagonists, angiotensin-converting enzyme inhibitors, diuretics).
For the treatment of mild to moderate depression in cardiac patients, the following doses of SSRIs are recommended: citalopram (cipramil) - 20 mg / day, paroxetine (Paxil) - 20 mg / day, sertraline (Zoloft) - 50 mg / day, fluoxetine (Prozac) - 20 mg/day, fluvoxamine (fevarin) - 50 mg/day. These doses are initial and at the same time can be considered as optimal therapeutic doses for mild to moderate depression. Thus, in most cases, a cardiologist or general practitioner does not need to titrate the dose when prescribing an SSRI. It is important to take into account some delay in the clinical effect of most antidepressants: a pronounced antidepressant effect of SSRIs is observed by the end of the first two weeks of therapy. It is advisable to inform the patient about this so that he does not expect an immediate positive effect from taking an antidepressant. In case of insufficient effectiveness, the above doses may be increased.
Drugs of the SSRI group have not only antidepressant, but also anti-anxiety (anxiolytic) effects, and therefore they are effective in patients with concomitant anxiety symptoms, panic attacks, and phobic syndromes. Some representatives of SSRIs also have a mild activating effect (for example, citalopram, fluoxetine).
Side effects of SSRIs are minimal, especially when prescribed at recommended doses. When using SSRIs, side effects from the gastrointestinal tract (dry mouth, nausea, diarrhea), as well as drowsiness, headaches, dizziness, tremor, sweating, were observed. These side effects occur rarely, usually in the first two weeks of treatment, and are reduced on their own. In most cases, discontinuation of the drug is not required.
In terms of clinical effectiveness, milnacipran (Ixel), a selective serotonin and norepinephrine reuptake inhibitor, is close to SSRIs. This drug is characterized by, along with an antidepressant, a moderate activating effect. The sedative effect is slightly pronounced. Therapeutic doses of milnacipran for the treatment of mild to moderate depression are 50-100 mg/day.
The tetracyclic antidepressant mianserin (Lerivone) is considered a “mild” antidepressant. Its mechanism of action is the blockade of presynaptic adrenergic receptors; the drug also blocks serotonin and histamine H1 receptors. Mianserin has mild antidepressant as well as pronounced sedative effects. Prescribed at bedtime in a dose of 15-60 mg. At therapeutic doses, the anticholinergic side effects of mianserin are less pronounced than those of tricyclic antidepressants.
In recent years, in addition to SSRIs, tianeptine (Coaxil) has been widely used in cardiological practice. According to the chemical structure, the drug belongs to atypical tricyclic antidepressants, and according to the mechanism of action it is a selective serotonin reuptake stimulator. Tianeptine has antidepressant, anti-anxiety and activating effects, and is safe in the treatment of depression in the elderly and patients with cardiovascular pathology. The drug does not cause clinically significant orthostatic hypotension, does not affect blood pressure, heart rate, blood sugar levels and other hematological parameters [7]. Tianeptine is recommended at a dose of 37.5 mg/day (1 tablet 3 times a day), if necessary, the dose can be increased to 50 mg/day.
Treatment of comorbid depression in cardiac patients, especially in patients who have undergone acute coronary incidents and heart surgery, not only leads to an improvement in mental state, but is also accompanied by positive dynamics in the clinical and functional status of patients, improves the quality of life, reduces the time of rehabilitation and return of patients to labor activity. It should also be assumed that treatment of depression in patients with coronary artery disease has a positive effect on prognosis. Currently, two large studies are being conducted - SADHART and ENRICH. Apparently, in a few years it will be possible to more definitively answer the question: does treatment of depression after myocardial infarction help reduce mortality in patients with coronary artery disease?
For questions about literature, please contact the editor
Is it true that once started, depression medications will have to be taken for the rest of your life?
Treatment with antidepressants is not a quick process, and its duration largely depends on the severity of the person’s condition. Typically, the course of taking these drugs lasts at least six months. If no improvement is observed after six months, the doctor may extend the course or change the drug. Therefore, you need to be prepared for the fact that you will have to take antidepressants for quite a long time. However, there is no talk of lifelong treatment: the patient will either begin to recover, and the need for antidepressants will gradually disappear, or will demonstrate resistance to them, and the doctor will choose a different strategy. There is also a WHO recommendation that antidepressants should be taken for at least another 9 months after the symptoms of depression have disappeared.
Does taking antidepressants guarantee a complete cure for depression?
Unfortunately no. Often, the causes of depressive disorders lie not in physiology, but in human psychology, so drug therapy has only a temporary effect, which gradually fades away after discontinuation of the drug. Knowing this, doctors usually use antidepressants as part of a comprehensive treatment that also includes psychotherapy sessions. However, some patients only need medication to recover. On the contrary, they don’t help some people. Depression is a very complex disorder, so doctors select their own treatment methods for each specific case.
Do antidepressants affect your sex life?
Strong antidepressants may indeed not have a very good effect on this delicate area of life in both men and women. Among the side effects of some tricyclic drugs is priapism - a prolonged painful erection not associated with sexual desire. More modern medications (MAO inhibitors) in some cases lead to anorgasmia and decreased sexual desire. This effect of antidepressants is considered a side effect, does not occur in everyone and disappears when the drug is discontinued and treatment is adjusted. Therefore, any sexual disorders that occur should be reported to your doctor immediately.
Are antidepressants compatible with alcohol?
During drug treatment of depression, alcohol consumption should be avoided. At the same time, different groups of antidepressants interact with alcohol in different ways. Mixing some (for example, tricyclic antidepressants) with alcohol can lead to serious consequences, including death. More modern drugs are not so dangerous in this regard. But MAO inhibitors, for example, can affect vasoconstriction (and therefore, indirectly, on erection), and can also enhance the effect of strong drinks and provoke, for example, damage to the liver or nervous system. Exactly how the drug interacts with alcohol is always written in the instructions. And in most cases this interaction is undesirable.
Sources
- Lacasse JR, Leo J. Serotonin and depression: a disconnect between the advertisements and the scientific literature // Florida State University College of Social Work, Tallahassee, Florida, United States of America PLoS Med. - 2005. - T. 2, No. 12.
- Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry 1998 // https://www.uptodate.com/contents/discontinuing-antidepressant-medications-in-adults/abstract/17
- Haddad PM Antidepressant discontinuation syndromes // Drug Safety journal. - 2001. - Vol. 24, no. 3. - P. 183-197. - PMID 11347722.
- American Psychiatric Association. The Truth About Antidepressants // https://www.psychiatry.org/news-room/apa-blogs/apa-blog/2016/03/the-truth-about-antidepressants
- Tarasenko O. A. Side effects of antidepressants // Pharmacist. - 2003. - No. 14. https://www.provisor.com.ua/archive/2003/N14/art_33.php
- WHO Mental Health Program // https://www.who.int/mental_health/mhgap/evidence/depression/q2/ru/
- F Hieronymus et al., Efficacy of selective serotonin reuptake inhibitors in the absence of side effects: a mega-analysis of citalopram and paroxetine in adult depression // Molecular Psychiatry, 2017; doi:10.1038/mp.2017.147
- Rafael Gafoor. Antidepressant utilization and incidence of weight gain during 10 years' follow-up: population-based cohort study // BMJ 2018; 361 https://www.bmj.com/content/361/bmj.K1951
- Megan Brooks. Antidepressants Worsen Sexual Dysfunction and Depression? https://www.medscape.com/viewarticle/870660
- Kucher E. O., Shevchuk M. K., Sivak K. V. Experimental study of the influence of alcohol on the biological effects of antidepressants // Bulletin of St. Petersburg University. – 2010. – Series 11, issue 1. https://vestnik.spbu.ru/pdf10/s11/s11v1_10_S.pdf
Dictionary
Psychopharmacology is the branch of medicine that deals with psychotropic drugs. Strictly speaking, the first were lithium preparations, which appeared in the 40s. But the generally accepted beginning of the era of psychopharmacology is the discovery of chlorpromazine (aminazine). Antidepressants are medications prescribed for depression and anxiety disorders. The most well-known groups of them are tricyclic antidepressants (for example, amitriptyline) and selective serotonin reuptake inhibitors (for example, Prozac). Amitriptyline is the most popular and famous antidepressant. A classic representative of the group of tricyclic antidepressants. Still in use today. Fluoxetine (Prozac) is the first selective serotonin reuptake inhibitor (SSRI). This name became a household name and entered into mass use. Prozac is good, first of all, not even because it is highly effective (in this regard, the old tricyclic antidepressants are still superior to it), but because it is well tolerated. SSRIs have made treatment available to the mass outpatient population. Of course, this immediately led to many excesses and abuses. Antipsychotics are drugs that have antipsychotic activity. With reservations, A. can be considered a synonym for the word “neuroleptic”, however, neuroleptics are a very clearly defined class, among them there is the so-called. “minor neuroleptics” (also known as “behavior correctors”), which actually have antipsychotic activity to a very moderate extent. Neuroleptics are potent antipsychotic drugs. Aminazine is the very first and most famous antipsychotic, the “gold standard”. In terms of potency, all other antipsychotics are measured in “chlorpromazine equivalent.” Phenothiazine is a heterocyclic compound, derivatives of which are widely used in medicine as neuroleptics, antihistamines and antiarrhythmics. Mood stabilizers are drugs that stabilize the emotional state and mood, eliminate mood swings and return behavior to normal limits (for example, lithium drugs). Psychotropic substances are any chemical compounds that affect mental processes. This phrase is often used as a synonym for drugs, but this is not entirely true. Psychopharmacological drugs are psychotropic substances. Caffeine (aka theine) is also a psychotropic substance. Stimulants are substances with a psychostimulating effect, almost all of which are prohibited for use. The classic representative is amphetamine. Tranquilizers are psychotropic drugs with a sedative, inhibitory, suppressive and hypnotic effect. In the classical sense, “major tranquilizers” are neuroleptics, but now tranquilizers are almost always understood as “minor tranquilizers,” that is, anti-anxiety, sedative and hypnotic drugs - first of all, this is a group of benzodiazepines (the most popular among which are phenazepam and diazepam) . Chlordiazepoxide (Elenium) is the first benzodiazepine tranquilizer. Currently, it is practically not used, since it has been replaced by subsequent drugs. Diazepam is the most popular benzodiazepine. The most popular tranquilizer and psychotropic drug in general. A classic non-nodiazepine tranquilizer, with all the pros and cons of its class. Acute psychoses are rapidly developing mental disorders. “Madness” or “insanity” in the common sense. GABA (γ-aminobutyric acid) is the main inhibitory neurotransmitter in humans. fMRI (functional magnetic resonance imaging) has made it possible to study the activity of living nervous tissue. The introduction of fMRI technologies has determined the progress of neuroscience in the last decade. The monoamine theory of action of antidepressants suggests that their medicinal effect is achieved through the specific blockade of certain receptors for monoamines - serotonin, norepinephrine, dopamine. The neuroplastic model does not negate the monoamine model, but evolutionarily develops and complements it. The neuroplastic model is one of the hypotheses for the mechanisms of action of antidepressants. It is assumed that their medicinal effect is achieved by influencing the processes of synaptic plasticity and the growth of neuronal terminals.