Molecular organization of myelin
A unique feature of myelin is its formation as a result of the spiral entanglement of glial cell processes around axons, so dense that there is practically no cytoplasm left between the two layers of the membrane. Myelin is this double membrane, meaning it consists of a lipid bilayer and proteins associated with it.
Myelin proteins include so-called intrinsic and extrinsic proteins. The internal ones are integrated into the membrane, the external ones are located superficially and therefore are weaker connected to it. Myelin also contains glycoproteins and glycolipids.
Proteins make up 25-30% of the dry matter mass of the myelin sheath of neurons in the central nervous system of mammals. Lipids account for approximately 70-75% of the dry mass. Spinal cord myelin has a higher percentage of lipids than brain myelin. Most of the lipids are phospholipids (43%), the rest are cholesterol and galactolipids in approximately equal proportions.
Stimulation of neuron myelination helps restore brain after stroke
Myelinating oligodendrocyte in rat brain
Wikimedia Commons
American scientists managed to achieve restoration of brain tissue after a white matter stroke in mice by stimulating the myelination of damaged neurons. The article was published in the journal Proceedings of the National Academy of Sciences
.
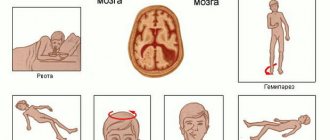
Ischemic stroke is an acute disruption of cerebral blood supply as a result of thrombosis, causing the death of a section of its tissue. There are two main forms of ischemic stroke. In the first type, caused by thrombosis of the large arteries of the brain, tissue damage affects large areas of the brain, including both gray and white matter. The second type of ischemic stroke is caused by small artery thrombosis and affects only the subcortical white matter. Over time, however, the damaged areas grow. About 25 percent of strokes are of this type. The likelihood of such a stroke increases with age and after 80 years it becomes almost one hundred percent. Such a stroke is the second most common cause of dementia (acquired dementia). Despite this, there is still no way to restore brain tissue after a white matter stroke.
At the first stage of the experiment, the authors, using a mouse model of white matter stroke, showed why brain tissue does not recover in this disease. A stroke causes demyelination of axons (damage to their myelin sheaths). This stimulates neurogenesis: at the site of damage, oligodendrocyte precursor cells (myelinating neuroglial cells) begin to divide. In nonischemic white matter injuries (eg, multiple sclerosis), progenitor cells then differentiate into mature oligodendrocytes, which remyelinate the damaged axons, resulting in partial repair of the damaged tissue. However, in white matter stroke, oligodendrocyte progenitor cells do not differentiate into mature oligodendrocytes but instead become astrocytes, leading to tissue gliosis (an proliferation of astrocytic neuroglia).
At the second stage of the experiment, the authors were able to reverse the blockade of maturation of oligodendrocyte precursor cells. To do this, they interfered with the Nogo protein receptor (NgR1) signaling pathway. This protein inhibits axon growth, and previous studies have shown that NgR1 receptor ligands are increased in brain tissue during stroke, while NgR1 inhibitors are decreased. It turned out that the introduction of an NgR1 antagonist into the damaged area of the white matter removes the blockade of oligodendrocyte maturation: the progenitor cells begin to mature more actively and no longer differentiate into astrocytes. This leads to myelination of damaged axons and tissue repair. In addition, in old mice with stroke, such therapy led to a noticeable restoration of motor activity - even in cases where the disease had already become chronic.
Recently, scientists demonstrated a way to restore brain tissue after a stroke by preventing inflammation: it turned out that receptor antagonists for the inflammatory cytokine interleukin-1 promote partial restoration of damaged tissue in rats. Previously, scientists were able to restore functions lost after a stroke by injecting modified mesenchymal stem cells into damaged areas of the motor cortex.
Sofia Dolotovskaya
Axon myelination
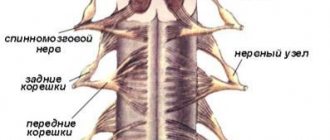
There are differences in the formation of the myelin sheath and the structure of myelin in the central nervous system and the peripheral nervous system.
Myelination in the CNS
Provided by oligodendrocytes. Each oligodendrogliocyte forms several “legs,” each of which wraps around part of an axon. As a result, one oligodendrocyte is connected to several neurons. The interceptions of Ranvier are wider here than in the periphery. According to a 2011 study, the most active axons in the brain receive powerful myelin insulation, which allows them to continue to work even more efficiently. The signaling agent glutamate[1][2] plays an important role in this process.
Myelination in the peripheral NS
Provided by Schwann cells. Each Schwann cell forms spiral plates of myelin and is responsible only for a separate section of the myelin sheath of an individual axon. The Schwann cell cytoplasm remains only on the inner and outer surfaces of the myelin sheath. Between the insulating cells there also remain nodes of Ranvier, which are narrower here than in the central nervous system.
The so-called “unmyelinated” fibers are still isolated, but in a slightly different pattern. Several axons are partially immersed in an insulating cell that does not close completely around them.
It has been established that late myelination of neurons, which continues in humans even into adulthood, greatly distinguishes them from chimpanzees and other primates[3].
White matter of the brain in the first month after birth
The microstructure of white matter required for the efficient and coordinated transmission of neural impulses undergoes particularly pronounced development in the first years of life, while deviations from this trajectory of brain development are likely to lead to changes in brain connections relevant to the behavior of the child and subsequently adult. Assessing white matter microstructure during normal brain development is critical to neurological approaches to both typical and pathological early development.
Research results reveal changes in the microstructure of white matter in the earliest periods of development and demonstrate different periods of regional development and regional asymmetries.
The neural architecture that forms bundles of myelinated nerve fibers, known as white matter microstructure, is fundamental to brain connectivity and contributes to higher-level cognitive functioning. The development of white matter structure (circuitry) occurs due to a cascade of complex processes such as axon formation, dendritic sprouting and myelination, which begins in late stages of pregnancy and continues to develop through childhood, adolescence and adulthood with the most pronounced maturation during the first two years life. During this developmental period, neural tissue substrates that govern individual differences in vulnerability or resistance to adversity are likely to begin to emerge early in brain development. Despite the importance of white matter microstructure for healthy brain function and connectivity, a significant gap remains in our knowledge regarding the normative characteristics of infant white matter microstructure, such as sexual dimorphism and asymmetry, especially in the weeks immediately following birth.
Magnetic resonance imaging (MRI) provides detailed images of the brain and can non-invasively monitor changes associated with white matter development. Diffusion MRI (dMRI) is very sensitive to changes in tissue microstructure and is widely used to study the fiber architecture of white matter. Diffusion tensor imaging (DTI) provides measurable measures of diffusion properties, including fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD), each of which provides quantitative measures of diffusion in brain tissue. Given the degree of anisotropic diffusion in white matter—due to tissue barriers such as axonal fibers and the myelin sheath—FA, MD, RD, and AD provide indirect markers of white matter microstructure.
More recent dMRI techniques, such as neurite orientation dispersion and density imaging (NODDI), use biophysical modeling to increase the level of detection of microstructural specificity available to neuroimaging. NODDI measures quantitative parameters of three specific diffusion processes: intraneurite diffusion (ν IC, diffusion within axons and dendrites), extraneurite diffusion, and isotropic (free) water diffusion (ν ISO). A similar model also quantifies the degree of neurite angular change through the orientation dispersion index (ODI). NODDI provides important information about brain development in infants and young children, with changes in parameters consistent with neurodevelopmental mechanisms of myelination and axonal fiber development during the first few years of life. For example, a nonlinear increase in the volume fraction of NODDI intraneurites (ν IC) occurs around the first 3 years of childhood, and ν IC continues to increase nonlinearly until 7.5 years. In clearly preterm infants, NODDI parameters indicate increased axonal dispersion and lower axonal density compared with timely born infants, and these parameters are also associated with poorer functional outcomes in very preterm infants. These studies provide valuable insight into brain development profiles and also demonstrate the utility of dMRI methodology.
Even within a narrow age range, measures of white matter microstructure vary by gestational age and exhibit broad left-sided differences. Sex differences were minimal, and no significant associations emerged between white matter parameters and neonatal growth markers. Finally, a comparison of DTI and NODDI values shows that although these methods measure similar aspects of white matter microstructure, they provide differential microstructural information early in child development. DTI diffusion indices are strongly associated with age adjusted for length of gestation, reflecting a decrease in total brain water content and an increase in membrane density. RD decreases and FA increases in central white matter regions, which may indicate early myelination or increased organization of white matter fibers and bundles. The advantage of NODDI is that it uses biophysical modeling to determine specific microstructural characteristics. ν IC is interpreted to provide an index of neurite density (i.e., axons and dendrites), whereas ODI is an index of the degree of fiber coherence.
Central white matter regions, such as the corpus callosum, internal capsules, and radials, develop in front of the more peripheral white matter, and the posterior regions develop in front of the more anterior ones, with most of the white matter microstructure being established after one month. Qualitatively, the FA and ODI maps at one month appear adult-like, suggesting that fiber architecture and organization are developing in the uterus. On the other hand, developmental processes such as myelination mainly occur postnatally and are likely to influence subsequent changes in RD and ν IC parameters
Notes
- Popov, Leonid
Biologists have revealed the secret of axon myelination. membrana.ru (August 11, 2011). Retrieved August 11, 2011. Archived February 15, 2012. - Hiroaki Wake, Philip R. Lee, R. Douglas Fields.
Control of Local Protein Synthesis and Initial Events in Myelination by Action Potentials. Science (4 August 2011). Retrieved August 11, 2011. Archived February 15, 2012. - Learning abilities linked to electrical insulation of neurons