Multiple sclerosis (MS) first became known to humanity as a separate nosology in 1868. Despite the fact that people have been dealing with this disease for such a long period, the causes of its occurrence and methods of treatment are still completely unknown (not a single case of multiple sclerosis has been completely cured).
Only since the end of the twentieth century have new drugs that modify the course of multiple sclerosis (DMMS) become available to patients, which, in certain types of the disease, can stop its development and preserve the patient’s ability to work.
Every patient diagnosed with MS has a question: what are the pros and cons of using DMTs?
What are PITRS
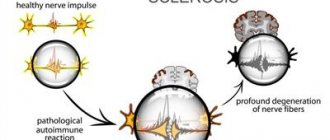
Since MS is an autoimmune disease, it involves a malfunction of the immune system.
Scientists have come to the conclusion that the basis of treatment should include immunomodulatory agents, the principle of which is based on the influence on the activity of the immune system. The DMT group includes medications containing the following active ingredients:
- interferon beta-1a;
- interferon beta-1b;
- glatiramer acetate;
- teriflunamide;
- fingolimod;
- mitoxantrone;
- natalizumab.
Monoclonal antibodies (MA)
The human immune system produces a large number of different antibodies to combat antigens of different types. The immune system's response to any of the antigens is polyclonal. Monoclonal antibodies are cloned cells synthesized in the laboratory.
Monoclonal antibody preparations have the ability to target antigens that need to be removed from the body. They attack myelin, bind to antigens and are subsequently eliminated from the body without causing harm. In addition, MAs activate the immune system against foreign antigens. When they enter the body, they “encourage” other components of the immune system to destroy target agents (for example, cancer cells).
Today in Israel, a new drug Lemtrada (alemtuzumab or Campath-1H) is successfully used to treat multiple sclerosis. Initially, it was developed for the treatment of cancer - leukemia and lymphoma. But the mechanism of action of the drug is such that it prevents the attack of myelin, thereby blocking new damage in multiple sclerosis.
Observations for 7 years of patients taking Lemtrada showed a general improvement and stabilization of their condition. The likelihood of relapse decreased by 50%, and the progression of disability by 42%. After taking Lemtrada, like other medications, side effects may occur. The most common of them:
- headache;
- rash, urticaria;
- fever;
- insomnia;
- upper respiratory tract infections;
- herpes;
- bleeding disorder;
- sinusitis.
In rare cases, problems with the thyroid gland may occur. But all side effects can be treated if you consult a doctor in a timely manner.
Studies confirming the effectiveness of immunomodulators
The first PMTRS was registered in 1993 and since then there have been significant breakthroughs in the treatment of the disease. New and highly effective medicines belonging to the DMT group are constantly being created. The wide range of drugs currently available has proven effectiveness, as confirmed by many international studies.
Studies of the safety and effectiveness of immunomodulators in the treatment of MS, including comparative studies, have been conducted since the late 1990s.
Key studies:
- After the FDA (Food and Drug Administration) approved betaferon (interferon beta-1b) in 1993 as a drug for the treatment of relapsing-remitting multiple sclerosis, a study was conducted over 16 years in eleven treatment centers in the United States to prove the effectiveness and safety of the use of this active substance in the treatment of relapsing-remitting type diseases. Throughout the study, there was a slowdown in disease progression and a decrease in the frequency of exacerbations.
- The BENEFIT (BEtaferon in Newly Emerging MS For Initial Treatment) study proved the appropriateness of prescribing betaferon after clinical isolated syndrome.
- When using the drug for two years, the risk of developing multiple sclerosis is two times lower. Modern medicine recommends prescribing betaferon after the first episode of the disease.
- A 2004 study at the Russian Medical University in Moscow, within the framework of a doctoral dissertation on the topic “Study of quality of life indicators and pharmacoeconomic analysis in patients with multiple sclerosis,” proved that the use of DMTs with such active ingredients as glatiramer acetate, interferon beta-1a, interferon beta- 1b prevents disability per year by an average of 45%.
- Pharmacogenetic studies based on pharmacogenetic analysis have received a new direction. In their course, the effectiveness of drugs is studied depending on the genetic characteristics of the patients. By identifying genes that influence the effectiveness of immunomodulators, scientists will be able to predict not only the clinical effects of an individually selected drug, but also predict possible adverse reactions. The result of the research will be the possibility of treating MS, taking into account individual characteristics.
However, it should be noted that no drug has yet provided complete recovery from the disease.
The medications currently being developed and introduced have a powerful and narrowly targeted effect on the course of neurological pathology. However, the expansion of drug therapy options has led to the emergence of new side effects and raised questions about the safety and tolerability of such medications.
This problem is especially acute when drug therapy must be continued continuously and for a long time to achieve and maintain the desired effect. In these cases, the favorable safety profile of the drug (risk/benefit ratio) plays perhaps the most significant role in maintaining a high level of patient adherence to treatment and achieving long-term effectiveness of therapy. Often, the presence of undesirable adverse reactions (ADRs) - any unintended and harmful manifestations for the human body that occur when using the drug in normal doses for the purpose of prevention, treatment and diagnosis [35] - minimizes the effectiveness of therapy due to reduced adherence or complete refusal of the patient from treatment. According to the most common classification of ADRs by the WHO Expert Committee, the following categories are distinguished: type A - dose-dependent; type B - dose-independent; type C - effects with long-term use (withdrawal syndrome); type D - delayed effects [11]. About 80% of all ADEs are type A and relate to the most commonly prescribed drugs.
The introduction of course-modifying drugs (DCMDs) into the treatment of relapsing-remitting multiple sclerosis (MS) has led to a transition from symptomatic treatment to pathogenetic therapy. DPIRS, according to data obtained in experimental and large-scale clinical studies (CTs), to one degree or another contribute to slowing the progression of the disease, reducing the severity and reducing the risk of exacerbations, and also has a positive effect on the dynamics of demyelination foci according to neuroimaging data [7].
As of September 2011, seven original drugs for the treatment of relapsing-remitting MS have been registered in the world, and about ten more drugs are in phase III clinical trials. Most of them require continuous use over an extended period of time. The “gold standard” drugs for first-line therapy currently include interferon (IFN) β-1b (Betaferon, Bayer), IFN β-1a for subcutaneous (Rebif, Merck Serono) and intramuscular administration (Avonex, Biogen Idec"), as well as glatiramer acetate (Copaxone, Teva). In Russia, in addition to them, a number of bioanalogs of original drugs are also registered - IFN β-1b (Extavia, Novartis; Ronbetal, Biocad; infibeta, Pharmstandard), IFN β-1a for subcutaneous (Genfaxon, MR Pharma SA") and IFN β-1a for intramuscular administration (Sinnovex, CinnaGen).
The spectrum of the most common side effects of IFN and glatiramer acetate therapy includes both injection site reactions and generalized systemic manifestations. However, it should be borne in mind that clinical trials are a kind of “pure” experiment; in a more complete form, the “landscape” of adverse reactions to a drug appears to the doctor only several years after its use in real clinical practice, as evidenced by the differences indicated in table
| Table |
[1, 6, 8, 10, 14, 16—21, 23, 25, 28, 30—32].
Local reactions
. Considering the injection method of using DMTs, the most common adverse reactions are reactions at the injection site - hyperemia, itching, swelling, pain, which can occur both at the time of drug administration and delayed. The pathogenesis of local reactions is based on the release of inflammatory mediators to the introduction of a foreign macromolecular object, however, not only the state of the body plays an important role, but also a number of characteristics of the drug (pH, needle diameter), injection technique, and also, probably, incomplete biological equivalence of the molecules [27]. Thus, according to phase III clinical trials, when using IFN β-1b 250 μg subcutaneously every other day, the incidence of this ADR was 69%, IFN β-1a 44 mg subcutaneously 3 times a week - 85%, IFN β-1a 30 mg intramuscularly 1 once a week - 33%, glatiramer acetate subcutaneously daily - 57% [18, 26, 31]. Reducing the severity of local reactions is possible by using appropriate injection devices and needles of smaller diameter, as well as local use of anti-inflammatory drugs and anesthetics. Necrosis at injection sites is relatively rare, their frequency does not exceed 1-3%, lipoatrophy in the form of local atrophy of subcutaneous fat is specific to glatiramer acetate - 15% [10] and in rare cases occurs when subcutaneous IFN is used [5].
Systemic reactions
. The most common systemic reaction to IFN drugs is a flu-like syndrome in the form of fever, chills, and headache. The pathogenesis of the development of this condition is associated with changes in the balance of cytokines and a direct pyrogenic effect on the central nervous system (hypothalamus) [9]. Often, especially with long-term use, systemic reactions to the administration of IFN can manifest as myalgia, weakness, mainly in the legs, and a feeling of fatigue. The incidence of influenza-like syndrome during therapy with IFN β-1b according to phase III clinical trials was 52–77%, IFN β-1a for subcutaneous administration was 45–69%, IFN β-1a for intramuscular administration was 53–76% [13, 16, 26]. At the same time, there is a connection between the severity of the syndrome and the patient’s age (it occurs in 60% of patients aged 18–28 years and only in 37% at the age of 42–50 years), with body mass index (the higher the body weight, the lower the likelihood of developing syndrome) and duration of use (on average, after 1 year of therapy, the frequency of influenza-like syndrome is 22-34%) [13, 16, 26]. Correction of the condition is achieved by taking prophylactic antipyretics (paracetamol, ibuprofen) and moving the injection time to the evening. Influenza-like syndrome is one of the most common reasons for discontinuation of DMT therapy [26].
Systemic adverse events such as depression and suicidal behavior also affect the quality of life of patients. It has been shown that the use of IFN drugs increases the risk of developing depression, while the use of glatiramer acetate does not lead to a similar effect [4, 6, 29].
For glatiramer acetate, the most significant systemic reaction to the drug is the post-injection reaction - chest pain, rapid heartbeat, anxiety, shortness of breath, difficulty swallowing; according to phase III clinical trials, the incidence of this condition was 15% [19]. The mechanism of the reaction is probably associated with a “cytokine storm” in response to the administration of glatiramer acetate and is the main reason for patients refusing therapy.
Paraclinical reactions
. Among paraclinical adverse reactions associated with the use of IFN, the most common are increased levels of liver enzymes, neutro-, lympho-, and, less commonly, thrombocytopenia. Changes in these indicators can serve as a criterion for the toxicity of a given dose of a drug.
An increase in the level of liver enzymes, primarily alanine aminotransferase (ALAT), less often aspartate aminotransferase (AST), in most cases does not have clinical manifestations and, according to phase III clinical trials, usually occurs in the first 12 weeks from the start of therapy. Thus, when using IFN β-1b, an increase in the level of ALT in the first 12 weeks was observed in 20%, and AST in 9% of patients; after 5 years, such deviations were observed only in 4 and 0% of patients, respectively [16]. During 6 years of phase 3 CT, IFN β-1a for intramuscular administration, a transient increase in the level of ALT and AST was detected in 44 and 27% of patients, respectively [14], and IFN β-1a for subcutaneous administration 44 μg - in 23 and 54%, respectively % [21]. Typically, an increase in ALT and AST corresponds to level 1 toxicity and does not require a change in the treatment regimen [33].
Changes in the hemogram are more typical for the long-term period of therapy: neutropenia, as a rule, occurs after the 1st year of therapy and lasts up to 5 years; Lymphopenia occurs later, but lasts longer. Despite the relatively common occurrence of these conditions in patients receiving DMT, they do not lead to clinical symptoms [34]. During the first 2 years of CT, a decrease in the level of leukocytes was observed in 20% of patients receiving IFN β-1a for subcutaneous administration at 22 μg, and 27% receiving IFN β-1a for subcutaneous administration at 44 μg [13].
With regard to glatiramer acetate, no similar changes in the activity of liver enzymes and a decrease in the content of blood cells were detected during CI and subsequent use of the drug [20].
Biosimilars
. Analysis of the literature to compare the side effects of original drugs and biosimilars is difficult, since there are only the results of individual studies on the bioequivalence of these drugs, which, due to the limited sample, does not allow us to draw definitive conclusions.
For the biosimilar IFN-β-1b extavia, we found data on three double-blind placebo-controlled clinical trials conducted in the USA, Canada and the European Union - in relapsing-remitting MS (n=372) [22], secondary progressive MS (n=718) [12] and clinically isolated syndrome (n=468) [21]. It was revealed that local reactions in the form of hyperemia and pain at the injection site occurred in 48-85% of cases, necrosis - in 1-5% of cases, while it was noted that the frequency of local reactions decreases with long-term use of the drug; Thus, after 6 months they were observed only in 21-47% of patients. Influenza-like syndrome occurred in 61-76% of cases, which is comparable to the original drug. Leukopenia and neutropenia while taking the IFN β-1b biosimilar were 82 and 18%, respectively, reaching only grade 1 toxicity; a significant increase in ALT was observed in 19% of patients. In the available literature there are no data from direct comparative studies of Extavia and the original drug IFN β-1b; according to the results of indirect comparison of placebo-controlled studies, the drugs do not have significant differences in profile.
According to the report, 36 patients participated in an open comparative multicenter study of the IFN β-1b biosimilar drug ronbetal (the reference drug is betaferon). At the same time, the incidence of influenza-like syndrome differed significantly: for example, the syndrome was registered in 31.4% of cases when using ronbetal versus 90.9% when using betaferon. Local side effects did not show significant differences: pain at the injection site against the background of ronbetal and betaferon - 14.3 and 9.1%, respectively, hyperemia at the injection site - in 62.7 and 81.8%, respectively. Paraclinical effects similarly did not differ.
An open-label study of the safety and tolerability of the biosimilar IFN β-1a for subcutaneous administration of Genfaxon at a dose of 44 and 22 μg included 68 patients [15]. When using the drug, adverse reactions were identified: flu-like syndrome was observed in 20.6% (n=14) of patients, hypersensitivity - in 7.4% (n=5), reactions from other systems (unspecified) - in 4.4 % (n=3). Local reactions (unspecified) were noted in 42.7% (n=29). No adverse reactions were detected in 54.4% of patients. When comparing this study with the PRISMS study [32] and FDA instructions for Rebif [24], it was found that flu-like syndrome when using Rebif occurred in 69% of cases (3 times more often than with Genfaxon), local reactions - in 72% (almost 2 times higher than when taking Genfaxon). At the same time, the frequency of hypersensitivity to the components of the drug was higher when using Genfaxon (for Rebif - 2%). Thus, the safety profile when comparing these drugs was quite different.
Information on the clinical trials of the drugs Infibeta and Synnovex is currently not available to the authors of the article.
The purpose of the study is a comparative analysis of DMTs and biosimilars.
Material and methods
Taking into account the difficulty of assessing the tolerability of biosimilars in comparison with original drugs according to clinical trials, also in order to study the safety profile of currently registered DMTs, we analyzed information from follow-up observations of patients who received such therapy in the Yaroslavl region in the period from 2009 to 2011. General the number of patients included in the analysis was 230, cases of DMT use were 401 (some patients had a history of using several drugs). The analysis included data on patients receiving IFN β-1a for intramuscular administration (Avonex - 3.0%), IFN β-1a for subcutaneous use (Rebif -19.2%, Genfaxon - 8.5%), IFN β -1b (betaferon - 16.5%, extavia - 18.2%, ronbetal - 18.0%), as well as glatiramer acetate (copaxone - 16.7%). Adverse events identified during questioning and examination of the patient were recorded, and subjective tolerability of the drug was also assessed.
For statistical processing of the obtained data, taking into account the dichotomous division (there is an effect/no effect) and the nonparametricity of the sample, Fisher's exact test was used.
results
In general, it should be noted that the drugs are well tolerated with long-term use: only in 21.9% of cases, DMT therapy was unsatisfactorily tolerated, in 10.7% of cases - poorly tolerated. At the same time, Copaxone was significantly (p<0.01) better tolerated than IFN (only in 4.5% - unsatisfactory and 1.5% - poor). Glatiramer acetate therapy was rarely interrupted due to side effects, even in the case of a specific generalized systemic reaction, which occurred in 6.0% of patients. This may be due to the better profile of the drug’s effect on the psychoemotional sphere in the form of a decrease in the severity of asthenia and fatigue, and the absence of a depressogenic effect [3].
While using Copaxone
(n=67) in patients, local reactions prevailed in the form of hyperemia (23.9%) and induration (19.4%) at the injection site, pain (4.5%), lipodystrophy (7.5%) and itching were less frequently recorded (6.0%) at the injection site. At the same time, pain and hyperemia at the injection site were noted significantly (16.5%, p<0.05 and 35.6%, p<0.01, respectively) less often than in the IFN group, and lipodystrophy was significantly more often (0.9 %, p<0.01).
When using the low-dose IFN β-1a Avonex (n=12), patients had predominantly generalized adverse reactions in the form of a flu-like syndrome (58.3%), while their frequency did not differ significantly from the IFN group as a whole. No moderate or severe local adverse reactions were recorded, which is likely due to the intramuscular route of administration. Good tolerability of Avonex was noted in 75% of patients, which did not differ significantly from the entire IFN group.
When analyzing the tolerability of IFN β-1a for subcutaneous administration (Fig. 1)
| Rice. 1. Comparison of side effects of Rebif and Genfaxon (% of patients). |
| ]]> |
It was found that the proportion of patients with moderate and poor tolerability of Rebif (n=77) was 24.7% (20.8 and 3.9%, respectively) - significantly lower than in the IFN group as a whole, and in the Genfaxon group (n= 34) this figure reached 70.6% (58.8 and 11.8%, respectively) - significantly higher than for the entire IFN group.
Influenza-like syndrome occurred in 33.8% of patients in the Rebif group (n=77), which did not differ significantly from the general IFN group. Systemic reactions also included cramps and weakness in the legs (7.8%), the frequency of these ADRs was the same as in the IFN group as a whole. Local reactions when using the drug were manifested mainly by hyperemia (35.1%) and compactions (11.7%) at the injection site, which did not differ significantly from the indicators of the group as a whole. A significant (p<0.001) lower incidence of pain at the injection site (2.6%) compared to the general IFN group deserves special attention, which may be due to the new drug formula and the use of automatic injection devices.
In the Genfaxon group (n=34), flu-like syndrome was observed in 61.8%, and cramps and weakness in the legs after injection - in 20.8% of cases, which was significantly higher than in the IFN group as a whole (p<0.01 and p<0.05, respectively). Hyperemia at the injection site occurred in 58.8%, pain in 47.1% of cases, which was significantly (p<0.01) more common than in the general IFN group. Seals at the injection site were detected in 23.5%, necrosis - in 2.9% of cases, which did not differ from the sample as a whole.
When comparing the frequency of ADRs for interferons β-1a for subcutaneous administration of Rebif and Genfaxon, it was revealed that the flu-like syndrome and local reactions in the form of hyperemia and pain at the injection site when using Rebif did not differ from the IFN group as a whole, and for Genfaxon this indicator was significantly higher .
In the IFN β-1b group (Fig. 2)
| Rice. 2. Comparison of side effects of Betaferon, Extavia and Ronbetal (% of patients). |
| ]]> |
satisfactory and poor tolerability of betaferon (15.2%) and Extavia (16.4%) was recorded significantly (p<0.01) less frequently than in the IFN group as a whole.
Analysis of the tolerability of ronbetal showed the opposite situation; unsatisfactory tolerability of the drug was recorded in 81.9% of cases (p<0.01). When assessing the spectrum of systemic adverse reactions, almost identical indicators were revealed for the Betaferon and Extavia groups: influenza-like syndrome occurred in these patients in 18.2 and 24.7%, respectively, which did not differ significantly from the IFN group as a whole. For ronbetal, the incidence of the syndrome was 59.7%, which was significantly higher than similar indicators for the group as a whole (p<0.01). Also in the ronbetal group, generalized skin rash (2.8%) and a feeling of stiffness and pulling cramps in the legs (13.9%) were more often noted, but no significant differences were obtained.
Local reactions in the form of hyperemia and pain at the injection site in the Betaferon (15.3 and 4.5%, respectively) and Extavia (19.2 and 4.1%, respectively) groups occurred significantly (p<0.01) less frequently than in the group as a whole.
In the ronbetal group, hyperemia at the injection site was significantly more often recorded - in 66.7% of cases (p<0.01), induration and swelling - in 31.9% (p<0.01), itching - in 8.5% ( p<0.05).
Discussion
It is believed that the original and generic drugs are identical to each other and have the same effectiveness and safety. Biosimilars are the most attractive in terms of price, which increases the availability of their use not only for the population, but also for the state. However, despite the economic advantages, biosimilars are rarely completely comparable to the original in terms of effectiveness and safety due to the impossibility of creating two absolutely identical cell banks used to obtain the active molecule and the impossibility of accurately repeating the long, knowledge-intensive production process. Any deviation, especially violation of cell culture conditions and simplification of the purification process, entails deviations in the structure of the final product and the ratio of isoforms; therefore, transferring a patient from one drug to another poses a particular risk [2].
Differences in the safety profile of individual biosimilars from the original DMTs identified according to the data from the MS registry of the Yaroslavl region (Fig. 3)
| Rice. 3. Comparison of drug tolerability based on patient survey data. |
| ]]> |
force us to once again draw attention to the need for a detailed study of the effects of drugs in the framework of clinical and post-marketing studies, including maintaining registers of patients receiving one or another DMT.
Conducting such studies is especially important due to the lack of sufficient convincing data on the comparative effectiveness of biosimilars and original drugs. Recently, WHO has been considering the possibility of introducing individual international nonproprietary names for each of the biosimilars, mandatory prescribing of biosimilars under a trade name or (in the absence of one) indicating the name of the manufacturer [2]. These measures are absolutely necessary to ensure monitoring of the immunogenicity, safety and effectiveness of biosimilars of DMTs after their registration on the pharmaceutical market.
Mechanism of action
Despite the general focus, the mechanisms of action of various drugs differ:
- Interferons beta have a general, nonspecific immunomodulatory effect.
- Glatiramer acetate has a specific effect: competitive replacement of myelin antigens at the sites of connection with molecules of the major histocompatibility complex. The result of the drug is inhibition of T1 lymphocytes and activation of T2 lymphocytes, the latter have a positive effect on the course of the disease and contribute to anti-inflammatory and neuroprotective processes.
- Teriflunomide inhibits the division of cells of the immune system, leading to a decrease in the number of circulating B and T cells. The drug has immunomodulatory and anti-inflammatory properties.
- Natalizumab is a recombinant monoclonal antibody against adhesion molecules. The action is aimed at slowing down the passage of lymphocytes through the blood-brain barrier, suppressing the activity of T-lymphocytes in areas of axonal damage and reducing the likelihood of recurrent inflammation in damaged areas.
- The action of Fingolimod is based on innovation: blocking sphingosine-phosphate receptors of lymphocytes, as a result of which lymphocytes do not leave the lymph nodes and, accordingly, the number of auto-aggressive T cells in the blood decreases; the drug also penetrates the brain and affects sphingosine-1-phosphate receptors of glial cells and neurons.
- Mitoxantrone creates bonds with nuclear DNA. The mechanism of its action is not fully understood. It is known that the drug has a cytotoxic effect on both proliferating and non-proliferating human cells, that is, its effect is not related to the phase of the cell cycle
Stem cell transplantation (SCT)
Expensive and quite effective technology today. The essence of the operation is to collect a bone marrow sample, after which the patient is prescribed a course of chemotherapy. The activity of the immune system decreases, and after this the previously taken stem cells are returned to the patient. Within 1-2 months, the patient’s immunity begins to fully function, significantly slowing down the process of myelin damage.
Stem cells regenerate white matter cells, eliminate scars in affected areas, and restore the function of nerve endings. In addition, stem cells have a positive effect on the body's immune system. Scientists believe that this method will further help defeat multiple sclerosis. The effectiveness of the technique is 50/50%.
PITRS first and second lines
As a rule, treatment of multiple sclerosis begins with first-line DMT:
In the initial stage of treatment the following is used:
- interferon beta-1a – subcutaneously (three times a week);
- interferon beta-1a – used intramuscularly (once a week);
- interferon beta-1b – subcutaneously (every other day);
- glatiramer acetate – subcutaneously (every day);
- teriflunamide tablets (every day).
The effect of first-line drugs has been studied better than the second. Their effectiveness is high, but in some cases insufficient, which gives the doctor reasons to prescribe second-line DMTs:
- Natalizumab – intravenous drip (once every 4 weeks);
- Fingolimod – tablets (daily).
To treat patients with high activity of relapsing multiple sclerosis, accompanied by rapid disability, when it is impossible to use alternative therapeutic agents, antineoplastic drugs are used (for example, Mitoxantrone - intravenous drip once every three months).
The main side effect of first-line DMTs is mental disorders manifested by depressive states. It is also possible that the drugs have a negative effect on the circulatory, hepatobiliary, and central nervous systems.
Second-line medications are characterized by several severe side effects - damage to the heart muscle, heart rhythm disturbances, and the appearance of progressive multifocal leukodystrophy.
When choosing second-line drugs, the doctor resorts to a careful analysis of the possible risks associated with their prescription. The patient is advised to undergo regular medical supervision.
Coimbra Protocol
The Coimbra protocol is a highly effective method that allows you to achieve long-term stable remission of the patient. The patient is prescribed a high dose of vitamin D, while foods containing calcium (cottage cheese, milk, etc.) are excluded from the diet. The norm for water consumption is established - at least 2.5 liters. This volume is enough to dissolve and remove calcium salts from the body. The visible effect of treatment occurs after two months, and after a year the patient’s condition enters the stage of stable remission. To maintain motor functions, the patient must systematically perform physical exercises.
The cost of treatment according to the Coimbra protocol is low, it only needs to be carried out under the supervision of a doctor. Significant improvement in the patient's condition is achieved in 95% of cases. In many ways, the result of treatment depends on the stage of the disease at which it was started. At stages 1-2, significant improvements are noted, but if treatment is started at a later date, the likelihood of stopping the progression of multiple sclerosis is quite high.
Therapeutic effect, indications
The goal of PED therapy is to cause the following changes in the patient’s body:
- reduce foci of damage to the central nervous system (brain and spinal cord) identified by MRI;
- reduce the number of exacerbations;
- alleviate the course of the disease;
- maintain efficiency and ability to self-care;
- delay disability;
First-line drugs are prescribed if the following symptoms are present:
- relapsing-remitting type of multiple sclerosis;
- age criterion: 18 - 50 years;
- the severity of the clinical picture does not exceed 5.5 points on the EDSS scale;
- over the previous two years, at least 2 exacerbations with a gap of 30 days or more between them and a stable neurological condition.
Indications for prescribing 2 line DMTRS:
- primary aggressive course of multiple sclerosis;
- insufficient effectiveness of first-line drugs.
In case of continuous deterioration of autoimmune processes against the background of specific treatment, antineoplastic agents (cyclophosphamide and mitoxantrone) can be used as third-line drugs.
In extreme cases, autologous bone marrow transplantation is used.
Treatment for exacerbations
An exacerbation of multiple sclerosis occurs when there is new damage to cells in the spinal cord or brain. This disrupts the transmission of nerve signals, which can cause new symptoms or worsen old ones. The exacerbation phase can last from a day to a month. Relapses differ both in nature and in severity.
Attacks may recur with varying intensity. Depending on the symptoms, the doctor may prescribe pulse therapy with corticosteroids - large doses of hormones administered intravenously, which will speed up recovery. As a rule, this is methylprednisolone or its analogues. To reduce the side effect, potassium supplements are prescribed, a diet high in potassium salts (apples, raisins, bananas), agents to protect the gastric mucosa (Almagel, Phosphalugel, etc.), as well as a course of antibiotics to exclude possible infection.
The use of human immunoglobulin droppers (Sandoglobulin, Pentaglobulin) is effective. The course of treatment is 5 days.
If it is impossible to carry out pulse therapy, treatment with Dexamethasone (intravenously or intramuscularly) is prescribed, with a gradual reduction in dosage.
In case of severe exacerbations, the doctor may prescribe plasmapheresis, a procedure for purifying blood plasma of antibodies. Plasmapheresis has been practiced since the 80s of the last century and shows good results. Treatment takes about 2 weeks, during which 3-5 blood filtration procedures are carried out through a special system. The patient's blood is separated into plasma and red blood (ermassa) using a special apparatus. Plasma with harmful substances is removed from the body, and the ermass is returned to the patient through another vein. Instead of plasma, albumin, donor plasma or its substitutes (hemodez, rheopolyglucin, etc.) can be administered. In some cases, pulse therapy and plasmapheresis may be combined.
With the increasing progress of multiple sclerosis and the ineffectiveness of hormones, a course of cytostatics (Azathioprine, Cyclophosphamide, etc.) is prescribed, which help suppress the autoimmune process. However, due to their high toxicity, these drugs have a number of side effects: a sharp decrease in leukocytes, red blood cells and platelets, and, as a result, drug-induced hepatitis develops, hair loss begins, and there may be frequent vomiting, diarrhea or nausea.
When medications are not used
The following contraindications are common to all PEDs:
- hypersensitivity to the active substance or auxiliary components of the drug;
- decompensated or severe forms of liver disease;
- pregnancy, breastfeeding.
Additional contraindications for interferons:
- primary progressive forms of MS;
- the presence of severe depression, thoughts of suicide, mental disorders;
- heart disease in severe forms.
Additional contraindications for glatiramer acetate:
- panic attacks.
Additional contraindications for teriflunamide:
- severe immunodeficiency states;
- hematopoietic pathologies;
- renal decompensation;
- acute infectious diseases in severe forms;
- a sharp decrease in protein in the blood;
- age up to 18 years.
Typical additional contraindications for second-line DMTs:
- immunodeficiency states;
- the possibility of infection of the patient with opportunistic microorganisms;
- cancer tumors, except basal cell carcinoma;
- progressive multifocal leukoencephalopathy;
- simultaneous use of first-line DMTs;
- childhood and adolescence.
You should know that the prescription of DMTs to children under 12 years of age must be approved by a medical commission; when choosing drugs, the results of diagnostic studies must be taken into account. Parents/legal guardians are informed of the possible risks and consent to prescribing medications to the child is obtained.
Diet for multiple sclerosis
In various sources you can find a huge number of articles and recommendations on nutrition for multiple sclerosis. Some of them strongly recommend adherence to special diets. Some experts claim that with diet you can be completely cured. However, this is not entirely true, although it is still worth excluding the use of certain foods. This primarily applies to animal products with high cholesterol content:
- fatty pork;
- fatty fish;
- sausages;
- offal;
- cheeses with high fat content;
- whipped cream;
- peanut butter and butter;
- eggs;
- goose fat;
- caviar;
- chocolate;
- cocoa.
Elevated cholesterol has a bad effect on vascular patency, which means that in MS it worsens problems with coordination of movements and reduces both physical and mental activity.
With multiple sclerosis, it is first of all important to adhere to a balanced diet in the ratio of proteins, fats and carbohydrates. You need to eat a large amount of fresh vegetables and fruits, foods high in microelements and vitamins. This promotes accelerated metabolism.
No less useful will be foods high in linoleic acid - nuts and vegetable oil, dishes and products made from whole grain cereals. To improve intestinal function, food should be high in fiber and plant fiber.
Keto diet . This is a low-carbohydrate diet, in which foods high in so-called carbohydrates are excluded from the diet. fast carbohydrates: sugar, sweets, flour dishes, baked goods, potatoes, pasta, etc. There have been isolated cases of the effectiveness of the keto diet for multiple sclerosis. In particular, a case is described with the restoration of a patient’s motor activity while following the Paleo diet, one of the varieties of the Keto diet.
Ashton Embry Diet . This is a special development of a Canadian scientist, which does not have fundamental evidence, but has collected a large number of positive reviews.
The basis of the diet is the exclusion from the diet of foods that can provoke the destruction of the body’s own tissues by the immune system. The diet includes a large number of substances that have a beneficial effect on the nervous system and prevent the development of disease. The basic principle is to take plenty of vitamins and minerals and eliminate foods that may cause a flare-up. Excluded from the diet:
- dairy products;
- all types of red meat;
- cereals containing gluten;
- beans;
- beer and brewer's yeast;
- beans;
- chicken egg yolks;
- sweets, including honey;
- strong tea and coffee;
- drinks and alcohol containing sugar;
- products containing palm or coconut oil;
- fast food.
It is recommended to include sea fish, gluten-free cereals, nuts, vegetables, and special vitamin complexes.
In any case, if you pay attention to your diet, there will be no harm to the body. Excluding foods that can harm the body from the diet is in the patient’s interests.
Practical application experience
According to the results of one of the scientific studies, “Patient Assessment of the Effectiveness of Multiple Sclerosis Therapy,” the results of which were published in 2021, the main indicators of the effectiveness of treatment are the absence of an aggravated clinical picture and progression of disorders of the nervous system.
At the same time, the subjective opinion of patients about the effectiveness of treatment was found more often than the effectiveness was confirmed by diagnostic methods. Over three years, the studied patients noted in 57% of cases a decrease in the number of exacerbations from 1 to 3 and in 24% of cases the absence of an aggravated clinical picture. All of these people find the therapy effective.
The study established a threshold for low treatment effectiveness: more than three episodes of exacerbation of the clinical picture in three years.
The study also found that subjective assessments of the positive effect of MS treatment directly depend on the relationship with the attending physician. Patients who indicated that their vision of the effect of treatment coincided with the goals set by the attending physician generally rated the results of therapy highly. Treatment was more often considered ineffective by those patients whose opinions differed from the goals of the doctors.
Permitted PITRS in Russia
Symptomatic treatment
This type of treatment is an addition to the main prescriptions for exacerbation of multiple sclerosis. Therapy is prescribed according to the clinical manifestations depending on the specific symptoms.
In the acute phase, patients often complain of spasticity, which manifests itself in stiffness of the affected limbs or parts of the body, and reflex spasms. They make it difficult for the patient to move and cause fatigue and immobility. Sleep disturbances or pain throughout the body are common.
To further preserve the functions of muscle tissue, it is important to continue to engage in physical therapy. Additionally, the doctor may prescribe physical procedures (electrophoresis, UHF therapy or ultraviolet irradiation) that help reduce inflammation of the joints and intoxication of the body, improve metabolic processes, and correct movement disorders.
There are general provisions according to which the doctor prescribes treatment:
- synthetic interferons (β-interferon);
- glucocorticoids. Prednisolone, dexamethasone, metypred or the hormonal drug ACTH;
- B vitamins, biostimulants, melin-forming drugs (Biosynax, Kronassial);
- cytostatics as a supplement: cyclophosphamide, azathioprine;
- muscle relaxants (mydocalm, lioresal, milliktin) are used to relieve muscle tone.