Very often, when describing the nervous system, “electrical” terms are used: for example, nerves are compared to wires. This is because an electrical signal actually travels along the nerve fiber. Each of us knows that a bare wire is dangerous, because it produces an electric current, and for this reason people use insulating materials that do not conduct electricity. Nature is also no stranger to safety precautions, and she wraps the nerve “wires” with her own insulating material - myelin.
Complex wrapper
Myelin surrounds the processes of nerve cells, insulating them from external influences. This is necessary for more reliable and faster signal transmission through the nervous system. Thanks to the insulation of the nerve fiber, the electrical signal does not dissipate and reaches its destination without interference. The speed of signal transmission along myelinated and unmyelinated fibers can differ by three orders of magnitude: from 70 to 140 m/s and from 0.3 to 0.5 m/s, respectively.
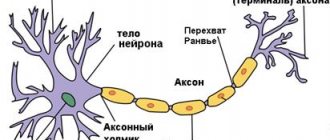
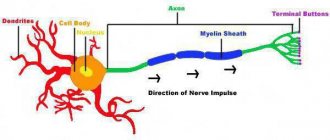
Essentially, myelin is the cell membrane of glial cells wrapped repeatedly around an axon. The membrane itself consists of 70–75% lipids and 25–30% proteins. In the peripheral nervous system, Schwann cells become membrane donors, and in the central nervous system, oligodendrocytes. These cells carefully wrap their membranes around valuable communication channels to ensure reliable communication between the nervous system and peripheral organs. Myelin does not cover the entire nerve fiber: there are gaps between the layers of myelin, called nodes of Ranvier (Fig. 1). There is a direct relationship between the distance from one gap to another and the speed of propagation of the nerve impulse along the fiber: the greater the distance between the nodes of Ranvier, the higher the speed of signal transmission in the nerve [1].
Figure 1. Nerve fiber wrapped in myelin. The nucleus of Schwann cells and nodes of Ranvier are visible - areas of the axon that are not covered by the myelin sheath.
theclickercenterblog.com
If we talk about the proteins that make up myelin, we must clarify that these are not only simple proteins. Myelin contains glycoproteins - proteins to which short carbohydrate sequences are attached. An important component of myelin is myelin basic protein (MBP), first isolated about 50 years ago. MBP is a transmembrane protein that can repeatedly “stitch” the lipid layer of a cell. Its various isoforms (Fig. 2) are encoded by a gene called Golli (gene in the oligodendrocyte lineage). The structural basis of myelin is an isoform with a mass of 18.5 kilodaltons [2].
Figure 2. Different isoforms of myelin basic protein (MBP) are created from the same gene. For example, to synthesize the 18.5 kDa isoform, all exons except exon II are used.
[2]
Myelin contains complex lipids called cerebrosides. They are the amino alcohol sphingosine combined with a fatty acid and a carbohydrate residue. Peroxisomes of oligodendrocytes take part in the synthesis of myelin lipids. Peroxisomes are lipid vesicles with various enzymes (in total, about 50 types of peroxisomal enzymes are known). These organelles are involved, in particular, in the β-oxidation of fatty acids: very long chain fatty acids (VLCFAs), some eicosanoids and polyunsaturated fatty acids (PUFAs). Since myelin can contain up to 70% lipids, peroxisomes are essential for the normal metabolism of this substance. They use N-acetylaspartate, produced by the nerve cell, to constantly synthesize new myelin lipids and maintain its existence. In addition, peroxisomes take part in maintaining the energy metabolism of axons [3].
Structure
The contents of the material substrate of the axon - axoplasm - contain very thin fibers - neurofibrils, and in addition microtubules, energy organelles in the form of granules, cytoplasmic reticulum, which ensures the production and transport of lipids and carbohydrates. There are pulpy and non-pulpous brain structures:
- The pulpy (also known as myelin or mislin) sheath of neutrites is found exclusively in representatives of the vertebrate species. It is formed by special lemmocytes (additional cells formed along the neutrites of the nervous structures of the periphery) “wound” around the process, in the middle of which the places unoccupied by the mislin membrane, the belt of Ranvier, are preserved. Only in these areas are voltage-gated sodium channels located and the activity potential appears again. In this case, the brain signal moves through the mislin structure in steps, which significantly increases the speed of its transmission. The speed of movement of the pulse along the neutrons with the pulpy layer is 100 meters per second.
- The pulpless shoots are smaller in size than the neutrites provided by the pulpy shell, which makes up for the losses in the speed of signal transmission in comparison with the pulpy branches.
At the site of union of the axon with the body of the neuron itself, an axonal eminence is located in the largest cells in the form of pyramids of the 5th shell of the cortex. Not long ago, there was a hypothesis that it is in this place that the post-connective capabilities of a neuron are converted into nerve signals, but this fact has not been proven through experiments. Fixation of electrical capabilities determined that the nerve signal is concentrated in the body of the neurite, or more precisely in the starting zone, at a distance of ~50 μm from the nerve cell itself. In order to maintain the strength of activity in the starting zone, a high content of sodium passages is necessary (up to a hundred times, with regard to the neuron itself).
Important wrapper
Myelination (the gradual insulation of nerve fibers by myelin) begins in humans already in the embryonic period of development. The first to pass this path are the subcortical structures. During the first year of life, myelination occurs in the parts of the peripheral and central nervous system responsible for motor activity. Myelination of areas of the brain that regulate higher nervous activity ends by the age of 12–13 years. From this it is clear that myelination is closely related to the ability of parts of the nervous system to perform functions specific to them. It is probably the active work of the fibers before birth that triggers their myelination.
The differentiation of oligodendrocyte precursor cells depends on a number of factors related to the functioning of neurons. In particular, working neuronal processes can secrete the protein neuroligin 3, which promotes the proliferation and differentiation of progenitor cells [4]. Subsequent maturation of oligodendrocytes occurs due to a number of other factors. In an article with the characteristic title “How Big is the Myelinating Orchestra?” describes the origin of oligodendrocytes in different parts of the brain [5]. First, in different parts of the brain, oligodendrocytes begin to mature at different times. Secondly, different cellular factors are responsible for their maturation, which also depends on the region of the nervous system (Fig. 3). We may have a question: are the oligodendrocytes that appeared with such a discrepancy in the starting data similar to each other? And how similar is their myelin? In general, the authors of the article believe that there are indeed differences between populations of oligodendrocytes from different parts of the brain, and they are largely due to the location of the cells and the influence of surrounding neurons on them. Yet the types of myelin synthesized by different pools of oligodendrocytes do not differ so much that they are not interchangeable.
Figure 3. Differences in the timing of oligodendrocyte formation in different parts of the brain and in the cellular factors influencing their development.
[5]
The process of myelination of nerve fibers in the central nervous system occurs as follows (Fig. 4). Oligodendrocytes send out several processes to the axons of different neurons. Coming into contact with them, the processes of oligodendrocytes begin to wrap around them and spread along the length of the axon. The number of turns gradually increases: in some areas of the central nervous system their number reaches 50. The membranes of oligodendrocytes become increasingly thin, spreading along the surface of the axon and “squeezing out” the cytoplasm. The earlier the layer of myelin was wrapped around the nerve ending, the thinner it will be. The innermost layer of the membrane remains quite thick - for metabolic function. New layers of myelin are wound on top of the old ones, overlapping them as shown in Figure 4 - not only on top, but also increasing the area of the axon covered by myelin.
Figure 4. Myelination of nerve fiber. The oligodendrocyte membrane wraps around the axon, gradually becoming denser with each turn. The inner layer of membrane adjacent to the axon remains relatively thick, which is necessary for metabolic function. Different parts of the figure ( a-c ) from different angles show the gradual winding of new layers of myelin onto the axon. The thicker, metabolically active layer is highlighted in red, and the new thickening layers are highlighted in blue. The inner layer of myelin (inner tongue in part b ) is covered by more and more layers of membrane, not only on top, but also on the sides ( c ), along the axon.
[6]
Myelination of nerve fibers by oligodendrocytes also significantly depends on the protein neuregulin 1. If it does not affect oligodendrocytes, then a myelination program is launched in them, which does not take into account the activity of the nerve cell. If oligodendrocytes received a signal from neuregulin 1, then they will then begin to focus on the work of the axon, and myelination will depend on the intensity of glutamate production and its activation of specific NMDA receptors on the surface of oligodendrocytes [6]. Neuregulin 1 is a key factor for triggering myelination processes in the case of Schwann cells [7].
Properties
The nutrition and growth of the axon depend on the body of the neuron: when the axon is cut, its peripheral part dies, while the central part remains viable.
With a diameter of several microns, the length of the axon can reach 1 meter or more in large animals (for example, axons coming from neurons in the spinal cord to the limb).
Many invertebrates (squid, annelids, phoronids, crustaceans) have giant axons hundreds of microns thick (in squids - up to 2-3 mm). Typically, such axons conduct signals to the muscles that provide the “flight reaction” (pulling into a hole, fast swimming, etc.). All other things being equal, as the diameter of the axon increases, the speed of nerve impulses along it increases.
Variable wrapper
Myelin is constantly formed and destroyed in the human body. The synthesis and breakdown of myelin can be influenced by environmental factors. For example, education. From 1965 to 1989, Romania was led by Nicolae Ceausescu. He established strict control over reproductive health and the institution of marriage in his country: he complicated the divorce procedure, banned abortion and introduced a number of incentives and benefits for women who had more than five children. The result of these measures was the expected increase in the birth rate. Along with the birth rate, the number of criminal abortions, which did not add to the health of Romanians, increased, and the number of abandoned children increased. The latter were brought up in orphanages, where the staff did not actively communicate with them. Romanian children have fully experienced what is called social deprivation - deprivation of the opportunity to fully communicate with other people. If we are talking about a small child, then the consequences of social deprivation will be a violation of the formation of emotional attachments and attention disorders. When the Ceausescu regime fell, Western scholars had to fully evaluate the results of the social policies of this dictator. Romanian children with severe problems with attention and establishing social contacts subsequently began to be called Ceausescu's children.
In addition to differences in the performance of neuropsychological tests, even the structure of the brain was different in Ceausescu’s children compared to children who were not in such conditions [8]. When assessing the state of the white matter of the brain, scientists use the fractal anisotropy indicator. It allows you to evaluate the density of nerve fibers, the diameter of axons and their myelination. The greater the fractal anisotropy, the more diverse the fibers that are found in that area of the brain. In Ceausescu's children, there was a decrease in fractal anisotropy in the white matter bundle connecting the temporal and frontal lobes in the left hemisphere, that is, the connections in this region were not sufficiently complex and diverse, with disturbances in myelination. This state of connections interferes with the normal transmission of signals between the temporal and frontal lobes. The temporal lobe contains emotional response centers (amygdala, hippocampus), and the orbitofrontal cortex of the frontal lobe is also associated with emotions and decision making. Disruption of the formation of connections between these parts of the brain and problems in their work ultimately led to the fact that children raised in orphanages experienced difficulties in establishing normal relationships with other people.
Myelination can also be affected by the composition of the food given to the child. With protein-energy malnutrition, there is a decrease in myelin formation. The lack of fatty acids also negatively affects the synthesis of this valuable substance, since more than 2/3 of it consists of lipids. Deficiency of iron, iodine and B vitamins leads to decreased myelin formation [9]. Basically, these data were obtained from the study of laboratory animals, but history, unfortunately, has given people the opportunity to assess the impact of lack of food on the developing brain of a child [10]. Hungry winter (Dutch hongerwinter) 1944–1945 in the Netherlands resulted in many children being born whose mothers were malnourished. It turned out that under conditions of starvation, the brains of these children were formed with disturbances. In particular, a large number of disorders were observed in the white matter, that is, problems arose with the formation of myelin. As a result, this led to a variety of mental disorders.
Damaged wrapper
Figure 5. Sensory impairment of the polyneuritic type. The name "sock-glove" is due to the fact that the anatomical areas corresponding to nerve damage are similar to the areas covered by these items of clothing.
website dic.academic.ru
It seems to me that the following rule is quite suitable for the human body: if there is an organ, then there must be a disease associated with it. In principle, this rule can be extended to molecular processes: if there is a process, there are also diseases associated with disruption of this process. In the case of myelin, these are demyelinating diseases. There are quite a few of them, but I will talk in more detail about two - Guillain-Barré syndrome and multiple sclerosis. In these disorders, damage to myelin leads to disruption of adequate signal transmission along the nerves, which causes the symptoms of the disease.
Guillain-Barré syndrome (GBS) is a disease of the peripheral nervous system in which the myelin sheath formed by Schwann cells is destroyed. GBS is a classic autoimmune disease. As a rule, it is preceded by an infection (often caused by the microbe Campylobacter jejuni). The presence of various pathogens in the human body triggers autoimmune damage to the myelin of nerve fibers by T- and B-lymphocytes. Clinically, this is manifested by muscle weakness, sensory impairment of the “socks-gloves” type (polyneuritic type) (Fig. 5). In the future, muscle weakness can increase until complete paralysis of the limbs and damage to the trunk muscles. Damages to the sensitive nervous system can also be varied: from a decrease in the ability to distinguish one’s own movements (impaired deep sensitivity) to severe pain. In severe forms of GBS, the main danger is the loss of the ability to breathe independently, requiring connection to a mechanical ventilation device (ALV). To treat GBS, plasmapheresis (purification of plasma from harmful antibodies) and intravenous infusions of human immunoglobulin preparations are currently used to normalize the immune response. In most cases, treatment leads to lasting recovery.
Multiple sclerosis (MS) is markedly different from GBS. First, this demyelinating disease leads to damage to the central nervous system, that is, it affects the myelin synthesized by oligodendrocytes. Secondly, there is still a lot of uncertainty about the causes of MS: too many genetic and environmental factors are involved in the pathogenesis of the disease. The fundamental point in the initiation of MS is a violation of the impermeability of the blood-brain barrier (BBB) for immune cells. Normally, brain tissue is fenced off from the rest of the body by this reliable filter, which prevents many substances and cells, including immune cells, from entering it. The BBB appears already in the embryonic period of development, isolating brain tissue from the developing immune system. At this time, the human immune system “gets acquainted” with all existing tissues, so that in the future, during adulthood, it does not attack them. The brain and a number of other organs remain “not presented” to the immune system. When the integrity of the BBB is disrupted, immune cells have the opportunity to attack unfamiliar brain tissue. Third, MS is characterized by more severe symptoms that require different therapeutic approaches. Symptoms depend on where the damage to the nervous system is located (Fig. 6 and 7). This may include unsteadiness of gait, sensory disturbances, and various cognitive symptoms. To treat MS, high doses of glucocorticoids and cytostatics are used, as well as interferon drugs and specific antibodies (natalizumab). Apparently, new methods of treating MS will be developed in the future, based directly on the restoration of the myelin sheath in damaged areas of the brain. Scientists point to the possibility of transplanting oligodendrocyte precursor cells or enhancing their growth by introducing insulin-like growth factor or thyroid hormones [11]. However, this is still ahead, and for now, more “molecular” treatment methods are not available to neurologists.
Figure 6. Lesions of the central nervous system in multiple sclerosis appear as white plaques on MRI.
website neurology.org
Figure 7. Depending on the location of damage to the nervous system in multiple sclerosis, there may be different symptoms: from tremor and ataxia with damage to the cerebellum to emotional disorders with localization of lesions in the frontal lobes.
www.diagnosisms.com
What is a nerve impulse?
This is the name for the excitation wave that spreads along the fibers as a response to irritation of neurons. Thanks to this mechanism, information is transmitted from various receptors to the central nervous system. And from it, in turn, to different organs (muscles and glands). But what does this process represent at the physiological level? The mechanism of nerve impulse transmission is that neuron membranes can change their electrochemical potential. And the process that interests us takes place in the area of synapses. The speed of the nerve impulse can vary from 3 to 12 meters per second. We will talk about it in more detail, as well as about the factors that influence it.
Literature
- Wu L. M., Williams A., Delaney A., Sherman D. L., Brophy P. J. (2012). Increasing internodal distance in myelinated nerves accelerates nerve conduction to a flat maximum. Curr. Biol. 22, 1957–1961;
- Harauz G. and Boggs J. M. (2013). Myelin management by the 18.5-kDa and 21.5-kDa classic myelin basic protein isoforms. J. Neurochem. 125, 334–361;
- Kassmann C. M. (2014). Myelin peroxisomes are essential organelles for the maintenance of white matter in the nervous system. Biochemie. 98, 111–118;
- Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S et al. (2015). Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. 161, 803–816;
- Tomassy G. S. and Fossati V. (2014). How big is the myelinating orchestra? Cellular diversity within the oligodendrocyte lineage: facts and hypotheses. Front. Cell Neurosci. 8, 201;
- Michalski J.-P. and Kothary R. (2015). Oligodendrocytes in a nutshell. Front. Cell Neurosci. 9, 340;
- Salzer JL (2012). Axonal regulation of Schwann cell ensheathment and myelination. J. Peripher. Nerv. Syst. 17, 14–19;
- Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M et al. (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 117, 2093–2100;
- Prado E. L. and Dewey K. G. (2014). Nutrition and brain development in early life. Nutr. Rev. 72, 267–284;
- Hulshoff Pol HE, Hoek HW, Susser E, Brown AS, Dingemans A, Schnack HG et al. (2000). Prenatal exposure to famine and brain morphology in schizophrenia. Am. J. Psychiatry. 157, 1170–1172;
- Bhatt A., Fan L. W., Pang Y. (2014). Strategies for myelin regeneration: lessons learned from development. Neural. Regen. Res. 9, 1347–1350.