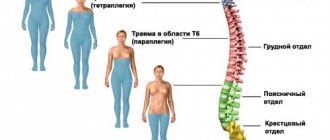
Indications and contraindications
Nerve stimulation is performed to relieve pain in the treatment of spinal pathologies
Transvertebral micropolarization of the spinal cord improves the general condition and eliminates pain during the treatment of the following diseases of the spinal cord and nerves:
- syndrome of unsuccessfully operated spine: spinal cord surgery can cause a condition characterized by persistent or recurrent radicular syndromes - pain, numbness, muscle weakness, etc.;
- radiculitis of the spinal region with or without pain;
- diabetic neuropathy;
- postherpetic neuralgia;
- peripheral nerve damage with the development of neuropathic pain syndrome;
- refractory angina;
- Raynaud's disease, obliterating endarteritis - vascular diseases of the lower and upper extremities;
- spinal canal stenosis.
A transvertebral neurostimulator is inserted after a spinal cord or spinal injury (severe bruise, etc.). The method is also effective in the treatment of spastic pain due to disruption of the pelvic organs and the consequences of a neurogenic infarction. Neurotechnology is used after surgery to remove a tumor on the back (in the spinal cord, another part).
Despite the effectiveness of the method, neuromodulation is prohibited if there are the following contraindications:
- development of severe somatic illness;
- incurable drug dependence;
- development of mental illness, history of suicide attempts;
- a decrease in the patient’s intellectual abilities, mental retardation (MDD), for example, with cerebral palsy, which is an obstacle to the use of an electrical stimulation system;
- the presence of an active infection in the area where surgery and the electrical stimulator are planned.
If such a surgical intervention is performed despite contraindications, it can cause negative consequences, including infection of the spinal cord, ineffectiveness of the intervention: lack of stimulation or its intermittency, change in the area of pain after surgery, etc.
Literature
1. Lance JW Symposium synopsis. In: R. G. Feldman, R. R. Young, W. P. Koella (ed)s. Spasticity: Disordered Motor Control .
Chicago: Year Book Medical Publishers; 1980; 485–494. 2. Mayer NH, Esquenazi A. Muscle overactivity and movement dysfunction in the upper motoneuron syndrome. Phys Med Rehabil Clin N Am. 2003; 14:855–883. 3. Zavalishin I.A., Osadchikh A.I., Vlasov Ya.V. (ed.). Upper motor neuron syndrome. Samara: Samara department. Literary fund, 2005; 440. 4. Young RR Spasticity: a review. Neurology. 1994; 44(9):512–520. 5. Sheean G. Neurophysiology of spasticity. In: Barnes MP, Johnson GR, eds. Upper Motor Neurone Syndrome and Spasticity, Clinical Management and Neurophysiology. Cambridge: Cambridge University Press, 2001; 12–78. 6. Mayer NH, Herman RM Phenomenology of muscle overactivity in the upper motor neuron syndrome. Eur Med Phys. 2004; 40:85–110. 7. Dewald JPA, Rymer WZ Factors underlying abnormal posture and movement in spastic hemiparesis. In: Thilmann AF, Burke DJ, Rymer WZ, eds. Spasticity: Mechanisms and Management. Berlin: Springer-Verlag; 1993; 123–138. 8. Burke D. Critical examination of the case for or against fusimotor involvement in disorders of muscle tone. In: Desmedt JE, ed. Motor Control Mechanisms in Health and Disease. New York: Raven Press, 1983; 133–150. 9. Gracies JM. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve, 2005; 31:552–571. 10. Kostenko E.V., Batysheva T.T., Ryabukhina O.V., Petrova L.V., Boyko A.N. Modern methods of treating spastic muscle tone using botulinum therapy. M.: 2011; 110. 11. Gusev E.I., Gekht A.B. Spasticity. Russian Honey. Journal 1999; 7 (12): 567–572. 12. Dietz V. Spastic movement disorder: what is the impact of research on clinical practice? J Neurol Neurosurg Psychiat. 2003; 74:820–821. 13. Damianos E. Sakas; Brian Simpson; Elliot S. Krames Operative Neuromodulation Volume 1: Functional Neuroprosthetic Surgery. (Acta Neurochirurgica Supplementum 97) An introduction. 2007; X: 482. 14. Minassian K., Hofstoetter U., Tansey K. et al. Neuromodulation of lower limb motor control in restorative neurology. Clin Neurol Neurosurg. 2012; 114(5):489–497. 15. Liberson WT, Holmquest HJ, Scot D., Dow M. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil. 1961; 42: 101–105. 16. Shealy CN, Mortimer JT, Reswick JB Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967; 46:489–491. 17. Cook AW, Weinstein SP Chronic dorsal column stimulation in multiple sclerosis. Preliminary report. NY State J Med. 1973; 73:2868–2872. 18. Martin R., Sadowsky C., Obst K., Meyer B. Functional Electrical Stimulation in Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2012; 18(1):28–33. 19. Minassian K., Hofstoetter US, Rattay F. Transcutaneous lumbar posterior root stimulation for motor control studies and modification of motor activity after spinal cord injury. In: Dimitrijevic MR, Kakulas BA, McKay WB, Vrbova G., editors. Restorative neurology of spinal cord injury. Oxford University Press; New York, 2011; 226–255. 20. Bamford JA, Mushahwar VK Intraspinal microstimulation for the recovery of function following spinal cord injury. Brain Res. 2011; 194:227–239. 21. Hofstoetter U., Mayr W., Rattay F., Dimitrijevic MR, Minassian K. Society for Neuroscience; Washington, DC: 2011. Effects of transcutaneous spinal cord stimulation on spasticity electrophysiologically evaluated in spinal cord injured individuals. Program no. 808. 02. Abstract Viewer/Itinerary Planner. 22. Minassian K., Persy I., Rattay F., Pinter MM, Kern H., Dimitrijevic MR Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum Mov Sci. 2007; 26:275–295. 23. Cook AW Electrical stimulation in multiple sclerosis. Hosp Pract. 1976; 11:51–58. 24. Siegfried J., Krainick JU, Haas H., Adorjani C., Meyer M., Thoden U. Electrical spinal cord stimulation for spastic movement disorders. Appl Neurophysiol. 1978; 41: 134–141. 25. Waltz JM Chronic stimulation for motor disorders. In: Gindelberg PL, Tasker RR, editors. Textbook for stereotactic and functional neurosurgery. McGraw-Hill; New York: 1998; 1087–1099. 26. Sedgwick EM, Illis LS, Tallis RC, Thornton AR, Abraham P., El-Negamy E. Evoked potentials and contingent negative variation during treatment of multiple sclerosis with spinal cord stimulation. J Neurol Neurosurg Psychiatry. 1980; 43: 15–24. 27. Gybels J., van Roost D. Spinal cord stimulation for the modification of dystonic and hyperkinetic conditions: a critical review. In: Eccles J., Dimitrijevic MR, editors. vol. 1. S Karger AG; Basel: 1985: 58–70. (Upper motor neuron functions and dysfunctions. Recent achievements in restorative neurology). 28. Illis LS, Oygar AE, Sedgwick EM, Awadalla MA Dorsal-column stimulation in the rehabilitation of patients with multiple sclerosis. Lancet. 1976; 1:1383–1386. 29. Richardson RR, McLone DG Percutaneous epidural neurostimulation for paraplegic spasticity. Surg Neurol. 1978; 9: 153–155. 30. Siegfried J., Lazorthes Y., Broggi G. Electrical spinal cord stimulation for spastic movement disorders. Appl Neurophysiol. 1981; 44: 77–92. 31. Barolat G., Singh-Sahni K., Staas WE, Jr., Shatin D., Ketcik B., Allen K. Epidural spinal cord stimulation in the management of spasms in spinal cord injury: a prospective study. Stereotact Funct Neurosurg. 1995; 64: 153–164. 32. Dimitrijevic MM, Dimitrijevic MR, Illis LS, Nakajima K., Sharkey PC, Sherwood AM Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: I. Clinical observations. Cent Nerve Syst Trauma. 1986; 3: 129–144. 33. Dimitrijevic MR, Illis LS, Nakajima K., Sharkey PC, Sherwood AM Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: II. Neurophysiologic observations. Cent Nerve Syst Trauma. 1986; 3: 145–152. 34. Pinter MM, Gerstenbrand F., Dimitrijevic MR Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control of spasticity. Spinal Cord. 2000; 38:524–531. 35. Murg M., Binder H., Dimitrijevic MR Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. Muscle twitches – a functional method to define the site of stimulation. Spinal Cord. 2000; 38: 394–402. 36. Minassian K., Persy I., Rattay F., Dimitrijevic MR, Hofer C., & Kern H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve. 2007; 35(3):327–336. 37. Minassian K., Jilge B., Rattay F., Pinter MM, Binder H., Gerstenbrand F. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004; 42:401–416. 38. Rattay F., Minassian K., Dimitrijevic MR Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. Quantitative analysis by computer modeling. Spinal Cord. 2000; 38:473–489. 39. Ladenbauer J., Minassian K., Hofstoetter US, Dimitrijevic MR, Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans Neural Syst Rehabil Eng. 2010; 18:637–645. 40. Jilge B., Minassian K., Rattay F., Pinter MM, Gerstenbrand F., Binder H. Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp Brain Res. 2004; 154:308–326. 41. Edgerton VR, Harkema SJ Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev Neurother. 2011; 11 (10): 1351–1353. 42. Roy RR, Harkema SJ, Edgerton VR Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch Phys Med Rehabil. 2012; 93(9):1487–1497. 43. Vrbova G., Slawinska U. Summary of strategies used to repair the injured spinal cord. In: Dimitrijevic MR, Kakulas BA, McKay WB, Vrbova G., editors. Restorative neurology of spinal cord injury. Oxford University Press; New York, 2011; 93–133.
Mechanism of operation
The affected area does not conduct nerve impulses well - the device delivers them to the centers of the brain
Every person has experienced pain at least once in their life. They are often localized in the back area. If the precipitating disease is not treated, the symptom becomes chronic, affecting the psychological, emotional and physical state.
Not everyone is in a hurry to see a doctor if they have constant pain, fearing that full treatment will not help get rid of the discomfort. To reduce the intensity of pain in modern medicine there are many ways, for example, medications using strong analgesics. Some mental and nervous diseases are treated using verbal therapy - electrical stimulation.
How the implantation of temporary electrodes for neurostimulation of the spinal cord works has not been fully clarified to this day. It has been established that in case of neuropathic pain, spinal cord stimulation promotes local neurochemical changes in the processes, which causes suppression of neuronal hyperexcitability.
Relevant studies have proven the possibility of serotonin release upon stimulation and suppression of the activity of irritating amino acids - aspartate and glutamine. For ischemic pain in the legs and arms, the analgesic effect is due to the restoration of the normal process of supplying cells with oxygen. As a result, the tone of the vessels decreases, their lumen expands, and spasm is eliminated.
The neuropathic stimulator is equipped with the following system:
- pulse generator;
- control panel;
- electrodes;
- connecting wires.
The device is inserted under local anesthesia through a small incision
The generator is implanted percutaneously. The mechanism of action is similar to a radio frequency receiver, which is controlled from an external module. This is an implantable pulse generator, which is charged using a battery without contact. No surgery is required to replace the battery.
The remote control is designed to control the radio frequency receiver (turn it on and off). It contains an easily replaceable battery.
Electrodes are metal plates with a common lead (a catheter or a tapering paddle-shaped plate). Micropolarization can be carried out using bipolar and multipolar electrodes.
Percutaneous electrodes (with a catheter-shaped outlet) are implanted under local anesthesia, using sedatives in a minimal amount. This helps to optimize the process of introducing the stimulant and reduce the risk of neurotrauma.
Paddle electrodes are inserted into the spinal cord through a surgical incision followed by a laminotomy or laminectomy. General anesthesia is used during the operation.
Types of electrical stimulation
Restoration of the patient’s body during the rehabilitation period using electrical stimulation is carried out in several ways:
- functional electrical stimulation;
- epidural stimulation;
- intraspinal microstimulation.
The mechanism of action of functional electrical stimulation is associated with the use of courses of influence by electrical currents, which makes it possible to induce muscle contractions and movements necessary to perform specific tasks. Muscle activation occurs due to stimulation of motor points.
Functional electrical stimulation is the most common method of treating the consequences of spinal cord injury. The cutaneous method of placing electrodes is more often used, and therefore I call stimulation non-invasive.
If lower paraplegia (paralysis of both legs) is observed, the use of multi-channel devices designed to restore walking movements and train standing is required. Skin electrodes stimulate the peroneal nerve, quadriceps femoris, and gluteal muscles.
The microcomputer ensures that stimulating impulses are sent to these sections, which creates conditions for leg extension, holding in one position, flexion and extension of the ankle joints when initiating walking.
The technique of epidural electrical stimulation of the spinal cord involves applying special electrodes to the roots. The generator is implanted percutaneously in the lower abdomen or buttocks. Control takes place using the remote control.
If we compare the epidural method with the functional one, the latter involves temporary use, that is, in courses, and the first involves long-term use. The effect of epidural stimulation is aimed at reducing spasticity and the appearance of motor reactions in the limbs.
Intraspinal microstimulation is provided by embedding microelectrodes into the spinal cord. Clinical cases of invasive stimulation in humans have not been reported to date. For now, the method is used only on animals.
Surgical intervention to install a spinal cord electrical stimulator should be entrusted to a qualified doctor who has the appropriate skills. The outcome of the operation and the risk of negative consequences depend on this.
RCHD (Republican Center for Health Development of the Ministry of Health of the Republic of Kazakhstan) Version: Clinical protocols of the Ministry of Health of the Republic of Kazakhstan - 2014
History of the technique
The history of neurostimulation began several centuries ago when it was noticed that certain weak impulses could relieve pain. Initially, electric stingrays were used for this, which were used by the inhabitants of the Mediterranean.
The stimulation was superficial. The first implantation of an electrode into the spinal cord was carried out in 1967, and in 1981 a neurostimulator was released that could be implanted. The device was used to reduce chronic pain.
Stimulators consist of implantable electrodes, connecting wiring and a pulse generator. They serve not only to reduce pain, but also to minimize the negative symptoms of central nervous system diseases.
DBS (neurostimulation of the brain)
The procedure involves implanting electrodes into a deep structure of the brain for subsequent stimulation. Using the stereotactic method, the doctor calculates the coordinates of the desired part of the brain in order to safely place electrodes there.
Neurostimulation of the brain is used to treat:
- Central nervous system disorders (neurosis, inexplicable fear, symptom of chronic fatigue).
- Parkinson's disease.
- Dystonia.
- Essential tremor.
There are certain restrictions for using the stimulant:
- Moderate and severe hypertension.
- Tendency to bleed.
- Inflammatory processes.
- Frequent development of vascular crises.
- Polytropic extrasystole.
- Trophic ulcers.
- Feverish condition.
- Fever.
- Presence of a pacemaker.
The use of a neurostimulator cannot completely eliminate the disease, but it can significantly improve the patient’s condition.
Necessary Precautions
Patients using SCS or DBS systems should take precautions. The following procedures should be avoided whenever possible:
- X-ray studies.
- MRI and ultrasound.
- Electrocautery (in the area where the neurostimulator is installed).
When driving a car or operating various mechanisms, the device should be turned off, since any disturbances in stimulation may cause loss of control.
Household appliances do not affect the device, so a person can freely use a microwave oven, TV, washing machine, cell phone.
It is necessary to avoid contact with metal detectors, welding equipment and not to stay near high-voltage transmission lines for a long time, since in these situations the position of the stimulator can turn off or on on its own.
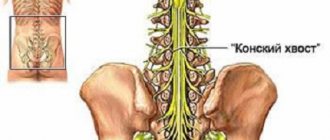
Spinal Cord Stimulation (SCS)
The electrode is placed between the bony walls of the spine and the dense outer meninges. The electrode is connected to a device (neurostimulator) implanted subcutaneously.
Indications for neurostimulation of the spinal cord are:
- Complications after spinal injuries.
- Motor and functional disorders.
- Chronic pain in the legs, arms, back, neck (neuropathic).
- Cerebral palsy.
- Spasticity (constant tension of any muscle group).
- Phantom pain.
- Angina pectoris (if drug treatment is not possible).
- Ischemic disease of the lower extremities (third and fourth degrees).
- Pain after damage to the plexuses or peripheral nerves.
There are also contraindications to the use of a stimulant:
- Local or general infection.
- Severe degenerative diseases of the spine that cause problems with electrode insertion.
- Pathologies of the immune system.
- Pacemaker implantation.
Before installing a permanent neurostimulator, test (temporary) stimulation is mandatory. During this time, both the doctor and the patient monitor the effectiveness of the device using external test electrodes. Usually this period lasts several days.
If the result is positive, surgery is performed to implant the stimulator. After this, the test period continues. At this time, the specialist changes the voltage parameters to establish the optimal stimulation mode.
Treatment
• intellectual disability of the patient that prevents the use of the system for neurostimulation.
Requirements for consumables Spinal cord neurostimulation system, consisting of two sets:
Patient kit:
• card with software.
Table 1 AP diagram
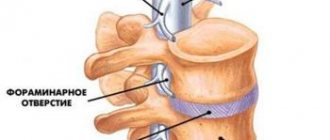
The choice of electrodes, their number, location along the spinal axis and in relation to the cross section of the spinal cord depends on the location and extent of the pain syndrome.
Figure 2 Implantation of a cylindrical electrode
Implantation of a flat electrode When implanting flat electrodes, the operation is performed under general anesthesia. Microlaminectomy is performed in the area of planned implantation. The electrodes are placed over the dura mater and fixed with anchors. Test intraoperative electrical stimulation is usually not performed.
Implantation of a sacral electrode
Indications for sacral electrode implantation:
Figure 3 Implantation of the sacral electrode.
Figure 4 Position of the sacral electrode.
Two types of stimulation are used: test and constant.
• stimulation parameters (amplitude, frequency, pulse width, polarity, combination of contacts).
Continuous stimulation is indicated in cases where, during test stimulation, a noticeable reduction in the severity of pain is achieved (usually by 50% or more from the initial level).
Using tunnelizers, the electrodes are passed through soft tissue into the area of the subcutaneous pocket (right/left gluteal region or left hypochondrium, taking into account the patient’s wishes), formed for the pulse generator. The electrodes are connected to the pulse generator.
Stage 3 Suturing the surgical wound Layer-by-layer suturing of the surgical wound is performed.
Possible complications when implanting a spinal neurostimulator electrode
• seroma at the site of neurostimulator implantation.
• short circuit, broken electrode/extension cord or loose connection in the circuit.
• ensuring high-quality fixation of stimulation system nodes intraoperatively.
| Vancomycin |
| Povidone - iodine |
| Cefazolin |
Information
Indication of absence of conflict of interest: none.
Indication of the conditions for revising the protocol: The protocol is revised at least once every 3 years, or when new proven data on diagnostic and treatment methods appear.
Spinal cord stimulation is a method of applying weak electrical impulses to the neural structures of the spinal cord to block various neurogenic pathological conditions.
The method of neurostimulation of the spinal cord is used for: treatment of spasticity and dysfunction of the pelvic organs, suppression of chronic pain syndrome (pain in the back and limbs, angina pain, rheumatoid arthritis, etc.)
Today, approximately 15,000 surgeries for implantation of SCS (spinal cord stimulation) systems are performed annually worldwide, of which 5,000 are performed in Europe. These figures show the great demand and significance of this method for patients. However, patient selection must be done with the utmost care and the decision to implant electrodes must be made in strict accordance with the selection criteria.
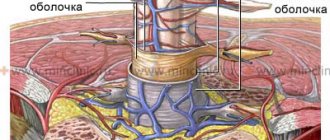
Neurostimulation is carried out using a small device-generator of electrical impulses, a special electrode that is implanted in the area of the spinal cord above the dura mater and connecting microwires. The entire system is not externally visible, since it is located under the skin and does not restrict the patient’s movements. The patient programmer is a hand-held remote control that allows you to adjust stimulation at your own discretion.
Based on medical indications, the patient is implanted with a system for chronic neurostimulation. Under X-ray control, a multi-contact electrode is implanted through a needle or through a small soft tissue incision. It is located above the membranes of the spinal cord, without touching the brain itself. The doctor determines the required electrical stimulation parameters and the accuracy of the electrode location. The electrode is connected to a subcutaneous programmable pulse generator using a thin extension cable. The procedure for implanting a neurostimulator does not lead to damage to the spine and spinal cord or disruption of nerve conduction through the spinal cord. The operation is performed under local anesthesia.
Before installing a permanent neurostimulating system, a trial (test) stimulation is required, during which the doctor and, naturally, the patient himself can verify the effectiveness of stimulation using an external test electrode. If a positive effect is achieved at this stage, a neurostimulator is implanted.
After implantation of the electrode, the trial stimulation period can last several days. At this time, the correct stimulation mode is selected by changing the voltage parameters of the pulse generator and the duration of continuous operation.
The main advantage of the neurostimulation method over all existing methods, in addition to its greatest effectiveness, is its reversibility and the absence of side effects and clinically significant complications. All implanted parts of the system can be removed or completely disabled if the desired effect is not achieved. Unlike many medications, spinal cord stimulation does not affect liver or kidney function and does not cause drowsiness, disorientation, or nausea.
Ordinary household appliances do not affect the stimulator. The patient can freely use a cell phone or radio, microwave oven, computer, television and other household appliances. However, contact with welding equipment, high-voltage power lines, theft detectors and metal detectors should be avoided. In these situations, the position may change from on to off and vice versa, but the stimulation program mode will remain unchanged.
General selection criteria for neurostimulation are: ineffectiveness of conservative treatment, the patient’s desire and ability to cooperate, the patient’s ability to control the neurostimulation system, favorable results of psychological testing.
Contraindications: inflammatory processes in the area of the proposed operation, unfavorable psychological examination, presence of a previously implanted pacemaker.
Surgical treatment, as well as preoperative and intraoperative diagnostics and postoperative monitoring should be carried out using modern technologies and the latest advances in the field of neurophysiology, microneurosurgery, and neuromodulation.
Sincerely! Pesnya-Prasolov Svetozar Borisovich Neurosurgeon, Russian Scientific Center for Surgery, Russian Academy of Medical Sciences
Medicine offers several methods for treating chronic pain and central nervous system pathologies. One of them is neurostimulation. It helps to carry out therapy without medications.
The goal is to relieve pain, restore or improve performance, and improve the patient's quality of life.
general information
Spinal cord neurostimulation is an innovative and proven technique (first proposed in 1967) that can provide pain relief, improve quality of life, reduce analgesic use in patients with neuropathic pain, and improve urinary function. The effect is achieved using electrical impulses that are delivered by electrodes implanted into the epidural space (Figure 1) [5] [6] [7] [10] [16].
Figure 1 Types of electrodes
I. INTRODUCTORY PART