Etiology and pathogenesis
The disease is inherited in an autosomal recessive manner. It appears to be based on an inborn error of metabolism, but the primary biochemical defect has not yet been identified. Both sexes get sick equally often. S. N. Davidenkov (1936) believed that the complex picture of Myoclonus epilepsy is an expression of the peculiar pleiotropism of the gene for this disease. Parents of patients with Myoclonus epilepsy can be healthy (there are often cases of their consanguinity). In relatives of patients with M.-e. other diseases of c are also observed. n. With. - epilepsy, parkinsonism, chorea, mental retardation, alcoholism, etc. Isolates with a relatively high frequency of M.-e. Along with familial cases, there are many sporadic cases.
Classification
Typing of myoclonic epilepsy is carried out according to a complex criterion: the moment of development and the main clinical picture. In system. Accordingly, they talk about a group of types.
Benign myoclonic epilepsy of infancy
Occurs in 30% of all cases. Accompanied by low-specific neurological symptoms reminiscent of hyperkinesis and tics. With a mild course, it is problematic to suspect something is wrong, because involuntary movements imitate the child’s normal physical activity. The disorder covers the shoulder girdle, face, and arms. Intellectual development does not suffer. Inhibition of mental development accounts for up to 5% of all described cases. Manifestation of myoclonic epilepsy of infancy is observed in the period from 2 months to 3 years of life.
Dravet syndrome
Occurs in the first year of life. Reminiscent of the classic infant form described above. In children it leads to severe mental deficiency, which manifests itself as oligophrenia and severe mental retardation. Neurological phenomena are also pronounced. Untreated attacks occur with a frequency of up to several times a week. This does not always require intense brain stimulation.
Unferricht-Lundborg syndrome
It has a genetic, hereditary condition. Accompanied by severe neurological deficits and mental disorders. Manifestation occurs in adolescence, towards the end of puberty. Less common before the age of 10 years. The frequency of such cases is determined to be 10-15%.
Myoclonic epilepsy of adolescence
It is extremely rare in children under 16 years of age, although it is possible. A characteristic feature is the complete absence of neurological disorders, persistent deficits, and mental disorders. The main symptom is tonic-clonic paroxysms. That is, classic convulsive attacks. It is interesting that they develop against the background of a completely intact, clear consciousness (not counting rare exceptions).
Sometimes there are so-called absence seizures. They are not considered a separate form of the disease. This is another symptom within the clinical picture of myoclonic epilepsy. Without going into details, these are paroxysms that resemble typical seizures.
The classification is used by doctors to clarify the clinical picture, develop diagnostic tactics, and further therapy.
There is a dynamic classification based on the developmental assessment of the disorder. Accordingly, they distinguish:
- Progressive type. This form is accompanied by a gradual, steady increase in neurological deficits and mental disorders. As the pathological process progresses further, the risk of fatal complications arises. Treatment does not help in all cases. Progressive myoclonic epilepsy is difficult to treat.
- Stable shape. The clinic is always at approximately the same level.
- Remitting or regressing variety. Symptoms develop over a short period of time. Then they fade away. If you look at it from a global perspective, there is a gradual retreat of the disease and restoration of normal well-being.
Pathological anatomy
A postmortem examination reveals dystrophic changes in the brain, most pronounced in the dentate nucleus of the cerebellum, olives of the medulla oblongata, substantia nigra, striatum, thalamus (visual thalamus), and in different parts of the cerebral cortex. A characteristic, but not obligatory histopathological sign of M.-e. is the presence of one or two spherical amyloid inclusions mainly in nerve cells, which were discovered by Lafora (GR Lafora, 1911) and named after him. Lafora bodies are found not only in ganglion cells of the brain and spinal cord, but also in the roots of the spinal nerves, in peripheral nerves, skeletal muscles, liver, and spleen. Many nerve cells also contain significant accumulations of lipofuscin.
Clinical picture
In most cases, the disease begins at the age of 10-15 years. It is characterized by a combination of myoclonic hyperkinesis with epileptic seizures. In some patients, the first symptoms are myoclonus (see), and general epileptic seizures (see Epilepsy), usually nocturnal, occur later, often after a number of years. In other patients, only epileptic seizures are initially observed, without myoclonic hyperkinesis, which appears later. In addition to grand mal seizures with loss of consciousness, clonic-tonic convulsions, urinary incontinence, tongue biting, other manifestations of epilepsy may occur - petit seizures, absence seizures (a type of minor epileptic seizure), twilight states, etc.
With M.-e. myoclonic hyperkinesis is characterized by great diversity and has its own characteristics. Initially occurring clonic twitches are often isolated and can be observed in one muscle; The quadriceps femoris muscle contracts predominantly, with little motor effect. In the future, myoclonus becomes more and more common, affecting the muscles of the limbs, torso, and head, and their frequency and motor effect increase.
In most cases, myoclonus is observed at a fast pace, lightning fast, erratic, arrhythmic, asynchronous, and irregular. They decrease significantly and stop in a calm state, in a supine position, and are absent during sleep. They are intensified by voluntary movements and emotions, especially negative ones. The so-called sensoroclonic reactions - an unexpected bright light or loud sound causes a short-term myoclonic attack in the form of a sudden increase in muscle contractions in the limbs, torso, face with sharp general shudders.
Myoclonic hyperkinesis manifests itself to varying degrees on different days - it can subside significantly or, conversely, intensify, and therefore patients themselves note an alternation of “good” and “bad” days. Sometimes some patients experience psychotonic attacks described by Lundborg. They are expressed in the fact that, under the influence of emotional influences, the patient experiences short-term tonic tension of the entire muscles without loss of consciousness.
Rare combinations of myoclonic hyperkinesis with choreoathetosis have been described (see Hyperkinesis). There is a close connection between myoclonic hyperkinesis and epileptic seizures. Day after day, the increasing myoclonus gradually becomes especially pronounced, at the height of which the patient loses consciousness for a short time, clonic-tonic convulsions appear with tongue biting and urinary incontinence. After the end of the epileptic seizure, myoclonus immediately disappears, usually for one or two days, and then it appears again and gradually intensifies. Myoclonus epilepsy has a progressive course. Due to myoclonus, it is difficult and gradually becomes impossible for patients to walk and perform other voluntary movements; they cannot care for themselves or eat food on their own. Some patients experience mild cerebellar disorders (ataxia, intention tremor), muscle hypotonia. Sensitivity is preserved. No paresis or reflex disorders were noted. In the later stages of the disease, extrapyramidal immobility develops with a wedge, a picture of parkinsonism (see), while myoclonic hyperkinesis disappears.
With few exceptions, patients deteriorate mentally.
In the early stages of the disease, asthenoneurotic disorders are noted, later the patients become more and more lethargic, apathetic, interest in the environment decreases, memory deteriorates, intelligence and criticism weaken, at times lethargy is replaced by irritability, there are states of confusion with hallucinatory experiences, many develop severe dementia (see . Dementia). An electroencephalographic study (see Electroencephalography) often reveals paroxysms of high amplitude electrical activity. Light stimulation and hyperventilation increase paroxysmal electrical activity of the brain. A decrease in the content of mucopolysaccharides is often detected in the blood serum of patients. According to some authors, for M.-E. An increased concentration of arginine succinic acid in the blood is also characteristic.
Juvenile myoclonic epilepsy (JME) as a separate form of idiopathic generalized epilepsy with age-dependent onset was identified in 1969 by D. Janz [1], although there is evidence in an earlier work by this author, jointly with W. Christian, in which it was named “impulsive petit mal” [2]. In addition, there is the first description of the patient, which dates back to 1867 [3]. However, despite the long period of study of JME, there are still questions and unresolved problems regarding its diagnosis and treatment.
The article provides a comparative analysis of the results of our research in accordance with the works of other authors. The results of the study by V.A. were compared. Karlova and N.V. Freidkova [4] 72 patients with JME, as well as a new sample of patients over the past 5 years (20 people) with research materials from domestic and foreign authors relating to the same time period. The evolution of the problem in the aspects of clinical diagnosis, therapy, prognosis of the disease and social adaptation of patients with JME is considered.
The age of onset of this form of epilepsy in the literature is indicated with a wide range, which is associated with the unfixed point of onset of the disease. This is explained by the fact that the onset of the disease, as a rule, is myoclonus, to which patients, and often doctors, do not attach due importance. If in our previous studies an early onset was noted at 10 years of age, and a late onset at 26, now we have recorded a case of an earlier onset, namely at 9 years of age.
As for the genetic aspect of JME, in the works of F. Elmslic et al. [5] and later other authors [6-8] identified pathological genes - BRD 2
and
EFHCI
with a locus on chromosomes 6p21, 6p12-p11 (
EJM 1
) and 5q14 (
EJM2
) with a defect in one of the genes - myoclonin. In addition, the presence of widespread disorders at the microstructural level in the white matter of the brain was confirmed - in the frontal lobe and corpus callosum. The presence of these changes is associated with frontal cognitive dysfunction, which was noted by D. Janz [1], who was the first to describe this form of the disease. He also emphasized that, although most cases of JME are isolated (single cases), approximately 1/3 of patients have a family history of epilepsy. Data from P. Thomas et al. [9] indicate that in families of patients with JME, there is a segregation of patients with idiopathic generalized epilepsy, and approximately 5% of first-degree relatives have epilepsy.
According to our latest observations, epilepsy in the family was observed in 1 relative of the first degree and in 5 relatives of the first degree according to previously published studies. In 2014, a study [10] was conducted on clinical and genealogical data in probands with JME and their family members. We studied 13 unrelated families in which at least 2 members suffered from epileptic seizures. Neither maternal nor any other mode of inheritance was found. A heterogeneous phenotype was found in individuals of the second and third degrees of consanguinity: in families with JME, other forms of epilepsy are found, in particular with generalized tonic-clonic seizures (GTS) in individuals with late onset JME.
Here is our own observation.
An 18-year-old patient complained of seizures from the age of 11 - “the same as the mother’s” upon awakening, and a single generalized convulsive seizure (GSE) at the age of 13. For the last two years he has been taking Convulex 750 mg 2 times a day, there have been no attacks. The breakdown of clinical remission began with the occurrence of an attack “around sleep” (when falling asleep).
Born from a 23-year-old mother, labor was at term, induced. The patient's mother was previously treated with a diagnosis of JME: she dropped objects from her hands, and had morning twitches in her hands. As prescribed by a neurologist, she took Luminal for a year, and then phenytoin (diphenin) for three years, which naturally intensified the attacks. At the age of 20, she independently stopped taking prescribed antiepileptic drugs (AEDs), at the same time the attacks practically stopped: only twitchings provoked by psycho-emotional stress remained.
An examination of the patient's neurological status revealed mild Babinsky asynergia. MRI of the brain showed a large subarachnoid cyst in the left frontoparietal region, undoubtedly congenital. The previous EEG showed mild background disorganization, absence of photosensitivity and an epileptiform response to hyperventilation. At the same time, in the slow-wave sleep phase, epileptiform discharges of the acute-slow wave and spike-wave type were recorded, mainly arising in the frontal leads, local or sometimes generalizing. The latest EEG shows their significant reduction.
There are a number of features in this observation. Firstly, the mother of a patient with a clinical picture of JME at the age of 20, against the background of self-cancellation of AEDs (which, by the way, were contraindicated for her), only a rare psycho-emotional provocation of myoclonus remained. Secondly, in the daughter, the combination of morning myoclonus with isolated sleep-related generalized seizures (GSEs) provides a known basis for the diagnosis of JME. At the same time, we believe that this case can be qualified as an “erased” form of JME, since there were no characteristic shudders and falling of objects from the hands. It can be assumed that the mother had an “erased” form of JME. In principle, these observations support the existence of a problem of sleep-related myoclonus, perhaps outside of epilepsy.
As is known, JME has a characteristic clinical pattern: bilateral, often asymmetrical irregular arrhythmic jerk-like myoclonic twitching of the muscles of the shoulder girdle and the arms themselves of varying amplitude. At the same time, myoclonic twitches are largely ignored by patients, and therefore patients, as a rule, do not contact doctors with these complaints. However, they can hardly be called unconscious, since the doctor’s leading questions are followed by a description of these attacks to the patient. Rather, this phenomenon can be explained by the characteristic features of the psyche of patients (anosognosia) [1].
Another characteristic clinical manifestation of this form of epilepsy is a generalized myoclonic phenomenon - startle. Unlike physiological shudders, this manifestation of the disease in JME occurs not so much during sleep as during the period of awakening. In one of the patients we observed, shudders predominated when going to bed. This brings JME closer to awakening GTCS epilepsy, in which seizures can occur around sleep.
The combination of myoclonus in most cases with rare GTCS (60%) is confirmed. According to our latest observations, the average time for the onset of GTCS after the onset of myoclonus was about 4 years. The combination of myoclonus only with absence seizures remains a rather rare phenomenon: we previously observed only one case, over the past 5 years there have been no such cases in our observations.
The combination of myoclonus, GTCS, atypical absence and aura, which causes some diagnostic difficulties, is reflected in the following clinical observation.
Patient X.
, 29 years old, applied in July 2013. She is a psychologist by profession, but does not work.
The prenatal and postnatal anamnesis is unremarkable, heredity is not burdened, according to the mother, it was “squeezed out” during childbirth. Against the background of apparent health, but at the same time chronic lack of sleep, in April 2012, during a trip to work on a train, the first and only GTCS occurred with a preceding feeling of the unreality of what was happening (the patient could not describe it more accurately). By the time he contacted us, therapy had already been prescribed - Topamax 50 mg per day, Lamictal 100 mg 2 times a day. This caused a deterioration - the appearance of shudders, more often associated with sleep.
MRI on one of the axial basal slices in T2 mode revealed increased signal from the pole of the left temporal lobe. Sleep EEG is represented by episodes of generalized spikes and polyspike waves, single and/or extended to several seconds (in these cases, reminiscent of atypical absence seizure).
Considering that the patient had a history of surgery for bilateral polycystic ovary syndrome (the menstrual cycle is not disrupted), and the first-line drug of choice for the dominant clinical manifestation of myoclonus may be levetiracetam, it was decided to cancel Lamictal and add Keppra 500 mg 2 times a day. In September 2013, there was a second GTCS, which the patient amnesiced, but at the same time, apparently, she felt its beginning (she left the kitchen and went to bed). In addition, there is an isolated, difficult-to-identify state of derealization. The twitching decreased. The dose of Keppra was increased to 750 mg 2 times a day and Topamax 50 mg 2 times a day was added (taking into account the patient’s excess body weight). There followed an increase in the frequency of attacks up to one per week while awake, and the patient independently discontinued the AED. After which there was no GTCS, however, general tremors occurred during sleep at night. During 12-hour EEG monitoring, bilateral synchronous peak-wave and polypeak-wave discharges with a frequency of 3.5-4 and a duration of 3-5 s were recorded during periods of wakefulness and sleep.
This case initially presented diagnostic difficulties: the differential diagnosis was made between JME and phenocopy JME. Uncharacteristic, although possible, for JME was the onset of the disease with GSP, an increase in convulsive seizures during the course of the disease, and an EEG pattern largely represented by a correlate of atypical absence. Another feature that initially led to discussion of the diagnosis of JME was the appearance of myoclonus, usually associated with sleep, due to its appearance after the prescription of a combination of Topamax (prescribed due to the patient’s excess weight) with lamotrigine. Since both of these drugs, although rarely, can provoke the appearance of myoclonus, the mere fact that myoclonus occurred not after waking up, but during sleep, did not negate the diagnosis of JME. Finally, one more significant circumstance: the self-cancellation of the drugs coincided with the spontaneous remission of GSP, although the twitching persisted. Thus, this case demonstrates that myoclonus is the essence of the disease. As for GSP, it is obvious that the first of them was provoked by lack of sleep, and the subsequent appearance and increase in frequency of attacks must be regarded as the result of an ineffective or even paradoxical effect of AEDs.
Let's return to the diagnosis. The onset of the disease is from the age of 10, when short-term episodes of “freezing” and flinching were noted; the first attack that occurred on the train was apparently associated with sleep deficiency, and the generalized changes revealed by the EEG could indicate the idiopathic nature of the disease: JME refers to idiopathic epilepsy with a varying phenotype. At the same time, a patient with GSP has a difficult-to-identify aura, which also occurs in the form of an isolated manifestation; transformation of convulsive seizures from asynchronous to seizures of wakefulness are signs characteristic of symptomatic and, to a large extent, cryptogenic epilepsy. Already in the 1989 classification, a form of epilepsy was identified with syndromes that have signs of both focal and generalized. In the later proposed and still not accepted classification in the section “Epileptic syndromes and related conditions”, the above heading was removed [11, 12]. However, the new composition of the International League Against Epilepsy (IALE) Commission on the Classification and Terminology of Epilepsy, headed by I. Scheffer [13], recently proposed the introduction of a section “Unclassified epilepsies”, which includes cases not recognized as known electroclinical syndromes, or of unknown etiology. Thus, this case does not relate to specific electroclinical syndromes and can be classified as an unclassified form of epilepsy.
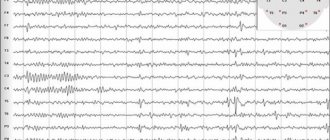
Since JME can initially manifest itself for a long time only as myoclonus, EEG is of particular value. EEG in JME is characterized by such features as the presence of short bursts of polyspike and spike waves of 3-6 v s (Fig. 1); discharges of generalized symmetrical spike waves 3 in s and rarely 1-2 in s; epileptic discharges characterized by high, sometimes gigantic amplitude. Frontal-lobar activity is detected more often (Fig. 2 and 3), sleep sharply activates epileptiform activity, while emphasis can also be detected in some other leads. Although clinically absence seizures were observed in isolated cases, during EEG recording, a correlate of absence seizures was recorded in 18.1% of early studies and in 15% of later studies, while an EEG correlate of myoclonus was recorded in half of the patients.
Rice. 1. EEG of patient Kh., 29 years old, with JME.
Rice. 2. EEG of patient X., 29 years old. Frontal lobar activity.
Rice. 3. Localization of the epileptic focus according to EEG leads (explanation in the text).
Focal EEG manifestations are widespread in patients with JME [14]. Thus, in the studies of E. Montalenti et al. [15], K.Yu. Mukhina et al. [16] revealed a high frequency of regional changes and asymmetry of diffuse peak-wave discharges. According to these authors, these “atypical” changes in the EEG are often the cause of an erroneous diagnosis of focal epilepsy with the phenomenon of secondary bilateral synchronization. In this regard, it is appropriate to mention that domestic authors emphasize: the classic clinical picture is fundamental in making a diagnosis, and EEG is just an auxiliary additional research method.
Unfortunately, despite the characteristic clinical picture of the disease and progress in research methods, the problem of diagnosing and prescribing correct therapy still remains open, although positive trends are observed. If previously a correct diagnosis was made and adequate therapy was prescribed only to 34.8% of patients, then according to recent studies, initial therapy was correctly selected for 50%. We note, by the way, that phenytoin is still sometimes prescribed.
The diagnostic problem is confirmed by some foreign works. Thus, a retrospective observation of 200 patients with JME who underwent outpatient examination in 2014-2015. [17], found an incorrect diagnosis in 49 cases with the onset of the disease with GTCS and registration of generalized spike-wave or polyspike-wave discharges on the EEG in 56% of cases. In cases where the disease began with myoclonic seizures, the diagnosis did not cause difficulties. Almost half of the patients were prescribed inappropriate AEDs; the rest were recommended to be monitored. Compared to the 1998 study, the rate of misdiagnosis has become lower and the time to correct diagnosis is shorter. However, diagnosis at a glance still remains a challenge among neurologists, even when typical EEG changes occur.
A substantiated diagnosis of JME, however, does not guarantee an optimal response to therapy. This fact was reflected in the work [18] on a study of 116 patients with JME, observed for at least 18 months each. 68 patients had no seizures over the past 12 months, and 48 had at least one seizure of a different type. The most common negative factors found in the latter group were: short follow-up period, medication other than valproic acid (VA), poor adherence to therapy. In particular, in this regard, the authors recommend wider use of valproate. Other researchers [19] noted that 19% of 201 patients with JME with no response to VC showed significant correlations and associations with mental disorders. This, as well as other treatment problems and causes of relapse are discussed in this article.
One of the problems in the treatment of JME, as with other forms of epilepsy, is adverse reactions.
Patient Z.
, fell ill at the age of 11-12, when twitching appeared in the morning after sleep, she dropped objects from her hands. MRI and EEG studies were carried out, after which the disorders were regarded as functional tics. At the age of 15, she suffered from salmonellosis, against the background of which a sharp headache developed, accompanied by vomiting, aggravated by verticalization. She underwent a course of treatment in the infectious diseases department, but the headache was not relieved, and a space-occupying process in the brain was suspected. In May of the same year, she was examined at the Scientific Center for Neurology, diagnosed with myoclonus and prescribed clonazepam and finlepsin. A positive effect of initial therapy was noted, but epileptiform activity remained on control EEGs. A year later, against the background of forced self-discontinuation of medications (the AEDs ended while on vacation), GTCS developed in the morning. The dosage of clonazepam was increased. Four years later, during the morning toilet in the bathroom, there was an episode of short-term loss of consciousness; there was no post-attack sleep.
At this time, the patient first contacted the authors of the article, and the treatment was changed: Depakine Enterik 300 mg 3 times a day was prescribed. The patient's condition improved significantly: no twitching was observed, and all subsequent EEGs were positive. However, adverse reactions appeared: weight gain, menstrual irregularities, hirsutism. It was decided to prescribe Keppra 750 mg 2 times a day and reduce the dosage of Depakine. Since the side effects of Depakine persisted, it was necessary to completely remove Depakine within a year, but after 3 months the EEG deterioration continued. When the Keppra dosage was increased to 2000 mg per day, the condition remained the same. During one of the attacks, the patient almost fell on the stairs; Depakine 300 mg/day was again introduced into therapy. A year later, a pronounced deterioration was recorded on the EEG; in agreement with the patient, it was decided to switch to combination therapy with Topamax 100 mg 2 times a day and Depakine Enteric 300 mg 2 times a day. The EEG began to show positive dynamics. Later, an attempt was made to remove Depakine, but the weakness in the arms increased, uncertainty appeared in the morning, eyes rolled, and negative dynamics were recorded on the EEG. It was decided to return to the dosage of Depakine 300 mg 2 times a day.
We present this case not to illustrate the side effects of VK, they are well known, but to show what difficulties can arise if it is intolerant. Perhaps we should have tried other forms of VC.
It is known that JME is more common in females in a ratio of 2:1. This reflects another side of the problem - the gender aspect. Despite the fact that valproates are effective first-line drugs for JME, in many cases they cannot be used in an effective dose in females of reproductive age due to severe side effects, as illustrated above. This problem was discussed by the European Academy of Neurology, and a recommendation was made to limit the use of valproate in this category of patients [20].
V. Puri et al. [21] tried to find the neurophysiological mechanism of the prevalence of morbidity in women using the method of transcortical magnetic stimulation in previously untreated patients. Increased activity was found in the cortical inhibitory neural network, “apparently due to a long period of cortical silence.” This phenomenon was found only in female patients, which the authors explain the increased sensitivity to this disease in the female cohort and, accordingly, the possible significance of the hormonal factor (estrogen).
In connection with the above, it is relevant to further search for an alternative drug VK for the treatment of JME in women.
Levetiracetam, which is highly effective against both myoclonus [22, 23] and in blocking interictal epileptiform discharges and photoparoxysmal response to EEG [24], can be a replacement for valproates in the treatment of JME.
In 2007, a study [25] was conducted on 30 patients treated with levetiracetam, of whom 80% achieved complete remission with levetiracetam monotherapy. In this case, the final therapeutic dosages ranged from 12 to 50 mg/kg per day. This study supports the possibility of considering levetiracetam as a first-line treatment for JME.
One of the authors of this article together with N.V. Freidkova [26] conducted a study that assessed the effectiveness of low doses of valproate and levetiracetam in the treatment of idiopathic forms of epilepsy; 13 of 23 patients had JME. At the same time, the group of patients with JME (both in combination with GTCS and without them) was the most representative in achieving the best therapeutic effect. Thus, complex therapy with the mentioned AEDs led to drug remission in 6 out of 8 patients; in 1 observation, the frequency of attacks decreased by more than 75%, and in another 1 - by more than 50%.
In another study [27] of the effectiveness of levetiracetam in monotherapy in 4 patients with JME (2 patients of whom were prescribed the drug for the first time in connection with pregnancy planning, the rest were transferred to carbamazepine therapy due to clarification of the diagnosis), the use of AEDs made it possible to achieve remission of attacks within 6 months
At the same time, according to some literature data [28], levetiracetam is not inferior in effectiveness to valproate. Domestic authors conducted an extensive retrospective study of a database of 1159 patients in the Volgograd region, of which 78 suffered from JME. The follow-up period ranged from 1 to 6 years. In 56% of cases, patients diagnosed with JME were on valproate monotherapy, in 17% of cases levetiracetam was used. The rate of achieving drug remission was 83%, while when using monotherapy with valproate - 92%, levetiracetam - 87%. Complete clinical EEG remission was achieved in 41% of cases, mainly with the use of valproate.
Also, along with the study of valproate, a prospective randomized study [29] was conducted on the use of lamotrigine in patients with the onset of JME in adulthood, which compared its effectiveness and tolerability relative to V.C. There is evidence that lamotrigine is effective and better tolerated in adult patients with JME, although the prevalence of idiosyncratic reactions may be a cause for concern.
It should be noted that modern scientific literature does not raise the question of the condition of children born to mothers with JME, as well as the role of male heredity. Let us give several examples from our practice, in particular in cases such as the one described below.
Patient L.
, 37 years old, first applied at the age of 18 after GSP, due to the fact that her menstrual cycle stopped (the attack was on the eve of the start of menstruation). The second GSP of sleep happened a week later, already against the background of menstruation. Subsequently there was only a single SHG. As for myoclonus, they recurred periodically. The patient complained of frequent disturbing dreams, for which reason phenazepam was prescribed. For the first time, when examining the patient, the EEG revealed acute-slow wave complexes of frontotemporal localization of an alternating type; the last EEG in a state of wakefulness showed an episode of generalized high-amplitude spike waves with maximum severity in the anterior parts of the brain. During all this time, the patient was pregnant three times, two pregnancies were terminated for social reasons, and one took place. As of January 2015, the child is 15 years old. He was distinguished by deviant behavior, a decrease in the level of intelligence; Until the age of 13, he had nocturnal and daytime enuresis, and studies in a special school.
Since the onset of the disease, the patient has been constantly taking Depakine Chrono 300 mg 2 times a day and lamotrigine (Lamolep) 100 mg 2 times a day. There are no convulsive attacks, but still shuddering in the morning.
It should be noted that the results of the study by K. Meador et al. [30] demonstrated a decrease in IQ (intelligence quotient) in school-age children whose mothers used VC during pregnancy by 7–10 points compared to other AEDs.
To date, a number of studies have been conducted on the long-term outcomes of using VC during pregnancy, the results of which indicate a 3-fold increase in the frequency of autism spectrum disorders and a 4-fold increase in autism in children, along with population indicators. Some studies give reason to believe that such children develop attention deficit hyperactivity disorder [31-33].
The effect of VK on the mental and physical development of children when used during pregnancy depends on the dosage, although the dose threshold has not been determined. This problem and a number of issues of pregnancy in JME against the background of VK monotherapy are discussed in detail in the article by P.N. Vlasov [34].
The presence of these problems is confirmed by the existence of cases such as the one given below.
Seizures occurred in a 6-year-old child born to a father with JME. The onset of the disease in my father began with a complex partial seizure in his sleep (automatism). He was prescribed initial therapy with carbamazepine (Finlepsin) 200 mg 2 times a day. Against this background, the patient, along with repeated complex partial seizures, developed attacks of “shuddering.” EEG showed regional and generalized spike and polyspike wave activity. Then, carbamazepine was gradually replaced by depakine 500 mg 2 times a day. Epileptic activity on the EEG regressed, and the twitching stopped. Two years later, taking into account clinical EEG remission, an attempt was made to reduce the dose of depakine to 600 mg per day with a drug concentration in the blood of 48.3-50.8 mcg/ml, however, absence activity appeared on the EEG during night sleep. When the dose was increased to 300 mg in the morning and 500 mg in the evening, there was a resumption of tremor in the hands. Complete remission was achieved with a dose of 1000 mg per day (500 mg per 2-fold dose). As for the child, the perinatal history, according to the parents, is not burdened, but it is observed by a neurologist with a diagnosis of “cerebral palsy, symptomatic epilepsy with rare convulsive seizures against the background of antiepileptic therapy.”
Unfortunately, genetic analysis was not performed in the described case, so the possible contribution of a genetic factor remains unproven. Another important, but unresolved issue for both the patient and his family members remains the issue of further prognosis of the disease.
In the work of J. Gaithner et al. [35], based on an analysis of patients observed for at least 25 years, unfavorable prognosis factors were identified: onset with GSP preceding myoclonus, failure of therapy and polytherapy. Contrary to conventional wisdom, long-term treatment is not necessary, except for those patients who have these unfavorable prognosis factors. At the same time, in our studies of 30 patients, discontinuation of AEDs after a 5-year drug remission was possible only in 9; in the remaining cases, a relapse occurred. It is possible that this phenomenon is to some extent associated with the late start of adequately selected therapy. According to our observations, over the past 5 years, there have been 3 cases out of 20 with complete clinical, drug and EEG remission during treatment with VK and/or levetiracetam.
In the study by K.Yu. Mukhina et al. [16] in 106 patients with JME, clinical remission was achieved in most cases (89.6%), and clinical EEG - in 22%. The majority of patients (79%) used monotherapy, the most frequently used was VK (56%), and less commonly, levetiracetam (13%). Unfortunately, despite the high effectiveness of treatment, the authors note a high percentage (92%) of relapses, most often at the stage of dose reduction by more than 50% and during the first year after discontinuation. The authors attribute this to such reliable factors as lack of sleep (23.5%), self-cessation, dose reduction or skipping AEDs, and replacement with generics (21%).
Difficulties in social adaptation, unusual lifestyle and poor cooperation with doctors in patients with JME were first mentioned by D. Janz and W. Cristian [2]. Similar changes, reaching the level of mental disorders, were registered in 25.6% [36, 37].
Since the peculiar mental disorders in patients with JME are associated with cognitive impairment due to the frontal lobes [20, 38], Brazilian authors undertook a special study in this direction on a contingent of 42 patients with an undoubted diagnosis of JME and 42 individuals in the control group using a battery of tests. The data obtained showed that patients with JME have worse adaptation in 2 significant aspects of life - work and family relationships, however, this factor is not correlated with cognitive impairment, but with a high frequency of attacks and impulsivity [39].
A significant factor for the patient and his relatives is the early prognosis of the disease. The authors of one of the studies [40] studied sensitivity to eye closure in JME and the effectiveness of prognosis in 76 patients with a minimum follow-up period of one year. 15.8% had a poor prognosis due to resistance to adequate AEDs, 19.7% were pseudo-resistant (i.e., mistreated), and 64.5% had a good prognosis. They also studied sensitivity to eye closure. It was found only in 4 (5.3%) patients and only in those with a poor prognosis. Thus, photosensitivity is likely to be a factor that worsens prognosis.
There are data in the literature [41] regarding the relationship between the photoparoxysmal response to EEG and optical coherence tomography data. In a group of patients with a photoparoxysmal response to EEG, an increase in the thickness of the retina nerve fiber layer
both eyes and the choroid of the right eye. The retinal thickness of both eyes was significantly less. The authors believe that these microstructural features may be associated with photosensitivity in patients with JME. Other studies [40] have shown that eye closure sensitivity (ECS) in patients with JME is a rare EEG finding and cannot be a reliable marker of poor prognosis.
It is known that a violation of cortical plasticity plays one of the main roles in the pathogenesis of JME. A group of Italian scientists [42] studied synaptic plasticity of the motor cortex using the method of paired associative stimulation in patients with JME and identified corresponding changes.
The data presented indicate positive changes in the diagnosis and treatment of JME. However, despite the characteristic clinical picture of the disease and the progress of modern research methods, as well as extensive clinical experience, the issue of diagnosing this form of epilepsy remains a problem for neurologists. Factors that aggravate the prognosis have been identified: a combination of myoclonus with GTCS, the presence of mental disorders, photosensitivity, lack of proper response to adequate AEDs. At the moment, the gender aspect of the disease still remains open, especially in relation to therapy. It is also necessary to accumulate scientific information about children born to mothers with JME and the role of paternal heredity.
The authors declare no conflict of interest.
Diagnosis
The diagnosis is made on the basis of a combination in a wedge, a picture of myoclonic hyperkinesis with epileptic seizures. The family nature of the disease is important for diagnosis. The differential diagnosis is made with a very similar cerebellar myoclonic dyssynergia of Hunt - a hereditary, autosomal recessive disease, in which myoclonic hyperkinesis and epileptic seizures are also observed, but, unlike M.-e., cerebellar disorders are much more pronounced. Unlike M.-e., with Kozhevnikov epilepsy (see), clonic convulsions are observed in a certain part of the body and are usually not of a generalized nature. It is especially difficult to establish an early diagnosis in sporadic cases when the disease manifests itself as an incomplete wedge, syndrome - either epileptic seizures or myoclonic hyperkinesis. In this case, family history is important, as well as a decrease in the content of mucopolysaccharides in the blood serum.
Treatment
Therapy is carried out using conservative methods. Treatment of juvenile myoclonic epilepsy requires the use of a group of medications.
New generation antiepileptic drug Levetiracetam
Valproic acid derivatives, specialized new generation antiepileptic drugs (Levetiracetam, as an example), barbiturates in small dosages, and benzodiazepine-based tranquilizers are used. Combination therapy is usually carried out, with valproate being the first line of treatment. Carbamazepines are used in older patients or when the patient reaches the age of 16-18 years, for the treatment of the disorder and its relapses in adulthood.
Treatment and Prognosis
Treatment is symptomatic. Anticonvulsant therapy is used, phenobarbital, benzonal, seduxen, chloral hydrate are prescribed. The last two remedies can significantly reduce myoclonic hyperkinesis for a short time. The use of chloral hydrate can cause addiction to it and the rapid development of cachexia. Repeated long-term courses of treatment with glutamine are also indicated.
The prognosis is unfavorable. Average duration of illness approx. 20 years, some patients live to old age. The most common cause of death is increasing cachexia, often pneumonia and other intercurrent diseases.
Spouses whose family has a patient with myoclonus epilepsy are advised to seek medical genetic consultation to resolve the issue of having a child (see).
Bibliography:
Davidenkov S.N. Myoclonus-epilepsy, in the book: Neurol. and genetics, ed. S. N. Davidenkova, vol. 2, p. 195, M., 1936; Dzerzhinsky V. Myoclonia Unverricht'a, Zhurn, neuropathist, and psychiatrist, book. 5-6, p. 293, 1910; McKusick V. A. Hereditary characteristics of man, trans. from English, M., 1976; Melnikov S.A. Myoclonus-epilepsy as a syndrome in certain diseases of the brain, Zhurn, neuropath, and psychiat., t. 57, century. 6, p. 740, 1957, bibliogr.; de Ajuriaguerra J., Sigwald J. et Piot C. Myoclonie-epilepsie familiare de type Unverricht, Presse med., p. 1813, 1954; Bogaert L. Sur l'epilepsie-myoclonie progressive d'Unverricht - Lundborg, Mschr. Psychiat. Neurol., Bd 118, S. 170, 1949, Bibliogr.; Davison Ch. a. Keshner M. Myoclonus epilepsy, Arch. Neurol. Psychiat. (Chic.), v. 43, p. 524, 1940; Gambetti P. ao Myoclonic epilepsy with lafora bodies, Arch. Neurol. (Chic.), v. 25, p. 483, 1971; Hallidau AM Les differents types des myoclonies, Rev. neurol., t. 119, p. 135, 1968; Handbook of clinical neurology, ed. by PJ Yinken a. G. W. Bruyn, v. 15, p. 121, 1974, v. 27, p. 171, Amsterdam ao, 1976; Lafora GR u. Glueck B. Beitrag zur Histopathologie der myoclonischen Epilepsie, Z. ges. Neurol. Psychiat., Bd 6, S. 1, 1911, Bibliogr.; LundborgH. Die progressive Myoclonus-Epilepsy, Upsala, 1903; Unverricht H. Die Myoclonie, L]3z.—Wien, 1891.
R. A. Tkachev.
How should the diagnosis of epilepsy be made?
According to the recommendations of the ILAE Commission (2001), the following five points should be adhered to:
- description of the paroxysmal event (possibly based on medical history);
- classification of attacks (history, visual observation, EEG);
- diagnosis of a form of epilepsy (clinical + EEG + neuroimaging);
- establishment of etiology (MRI, karyotype);
- diagnosis of concomitant diseases and degree of disability.
Based on etiology, there are three forms of epilepsy: idiopathic, symptomatic and presumably symptomatic (cryptogenic).
In idiopathic forms, there are no diseases that can cause epilepsy; it is an independent disease. Currently, the genetic determination of idiopathic forms of epilepsy has been established. The question often arises about how likely a child is to inherit generalized idiopathic epilepsy if one of the parents suffers from it. In fact, the probability of this is low, it does not exceed 8%.
By symptomatic forms we mean epileptic syndromes with a known etiology and verified morphological disorders (tumors, scars, gliosis, cysts, dysgenesis, etc.).
Cryptogenic (hidden) forms include forms whose cause remains hidden and unclear. These syndromes do not meet the criteria for idiopathic forms, but there is no evidence of their symptomatic nature; visualization does not detect structural changes.
Idiopathic generalized epilepsies (IGE) are the most common group of epilepsies in pediatric neurology. These are peculiar forms of the disease with a genetic predisposition. A genetic defect of many forms has been found: the pathogenesis of the development of the disease is based on channelopathies (the neuron membrane is unstable and the form of manifestation will be generalized, since all cells of the cerebral cortex are affected).
The following forms of IGE are distinguished:
- benign convulsions of newborns (familial and non-familial);
- benign myoclonic epilepsy of infancy;
- childhood absence epilepsy;
- juvenile absence epilepsy;
- juvenile myoclonic epilepsy;
- epilepsy with generalized convulsive seizures of awakening;
- primary photosensitivity epilepsy.
IGE includes various epileptic syndromes in which all seizures are generalized from the very beginning.
IGE should not reveal any other causes of the disease other than genetic factors, since this form is, by definition, idiopathic.