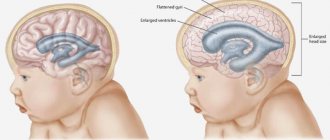
What is cerebral hypoplasia
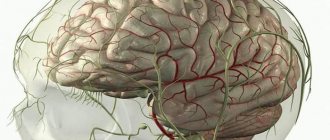
Hypoplasia of the brain is the formation of vessels and elements of the circulatory system that are lagging behind. An abnormal phenomenon that provokes ischemic lesions, destructive and atrophic processes. Often contributes to the occurrence of stroke and ischemia.
The left and right vertebral vessels form a blood pool, which transmits 20% of the blood volume. The carotid aorta is responsible for the remaining 80%.
As a result of circulatory disorders, parts of the head, such as the cerebellum, occipital lobes and trunk, do not receive enough oxygen. The patient's general health deteriorates and vascular insufficiency develops, which affects the entire cardiovascular system.
Cerebellar hypoplasia
Hypoplasia does not always affect the entire brain. In some cases, only some of its parts, such as the cerebellum, are affected. Cerebellar hypoplasia is a congenital malformation in which some functional areas of the cerebellum and/or its cortex are underdeveloped. Like any other type of hypoplasia, cerebellar hypoplasia occurs during fetal development. In more than half of all cases, the development of the disease is caused by genetic abnormalities. In other cases, the reason lies in the impact of certain negative factors on the body of a pregnant woman.
Classification of the disease
Vascular disease affects and damages all areas of the brain. They provoke slow tissue development. The pathology may be congenital or appear with age.
Hypoplasia of the cervical artery most often begins to develop in the fetus in the womb.
The circle of Willis ensures normal blood supply to the brain. It is formed by large aortas of the spine with two branches. Usually the vessels develop evenly. A branched artery is responsible for natural blood supply. Vascular hypoplasia can be left-sided, right-sided or bilateral.
Hypoplasia of neck vessels disrupts blood supply and prevents normal nutrition of brain cells.
If you do not undergo diagnosis in time, cardiovascular failure and serious malfunction of the vestibular apparatus may develop.
First, you should undergo certain therapy, due to which the blood flow will return to normal.
Right artery lesion
Hypoplasia of the right vertebral vessel is somewhat more common. Symptoms of hypoplasia of the right vertebral artery are similar to fatigue or malaise, the emotional background is unstable.
The right vessel in the spine is 2 times smaller than the left one, so if the lumen is narrowed, then the anomaly is not obvious.
The nutrition of the occipital region of the head is disrupted, so the emotional background will malfunction, and visual impairment is observed.
In rare cases, it affects the general condition of the patient, constant fainting, impaired coordination of movement, and dizziness after getting out of bed.
Hypoplasia of this side is dangerous because the resulting blood clot can block the lumen, which will lead to a stroke.
Hypoplasia of the left transverse sinus
Found (9 posts)
. , got the result: Brain without organic changes. Decetration of tooth C2. Unclosed circle of Willis, Hypoplasia
of the Left Transverse Sinus
. There is no evidence of an aneurysm or arteriovenous malformation. . open
During an MRI, my 6.7 year old child was diagnosed with Hypoplasia
of the central sections
of the Left Transverse Sinus
. We took him for an MRI after he had febrile convulsions 2 times at a high temperature of 39 and above. could you explain ours to us? open
. , where they identified “Multiple anomalies in the development of cerebral vessels. Hypoplasia
- aplasia of the V1 segment of the left vertebral artery.
Aplasia of the Left Transverse Sinus
, accompanied by congestion of the superficial veins. open
. ,S-shaped scoliosis of the cervical spine. Local stenosis of the insular part of the right middle cerebral artery. Hypoplasia
of the Left Transverse Sinus
. What do you think is the reason for this condition of mine? And is it really possible to come out of it when? open
. ECHO and REG showed nothing; an MRI of the head diagnosed: “Mild hypoplasia
intracranial part of the right vertebral artery and hypoplasia
the Left Transverse Sinus
,” on MRI of the cervical spine. open
. : main: G96. Multiple anomalies of cerebral vascular development. Hypoplasia
- aplasia of the V1 segment of the left vertebral artery.
Aplasia of the Left Transverse Sinus
, accompanied by congestion of the superficial veins. open
. : main: G96. Multiple anomalies of cerebral vascular development. Hypoplasia
- aplasia of the V1 segment of the left vertebral artery.
Aplasia of the Left Transverse Sinus
, accompanied by congestion of the superficial veins. open
. MRI: Moderate cyst-like expansion of the subarachnoid convexital spaces in the frontal regions. Inclusion of the right posterior communicating artery. Hypoplasia
of the Left Transverse Sinus
. I am 28 years old. I have been suffering severely since I was 25 years old. open
. MRI: Moderate cyst-like expansion of the subarachnoid convexital spaces in the frontal regions. Inclusion of the right posterior communicating artery. Hypoplasia
of the Left Transverse Sinus
. I am 28 years old. I have been suffering severely since I was 25 years old. open
Left artery lesion
The disease is not easy to recognize in the early stages. It develops for several years and does not make itself felt. Impaired blood circulation provokes stagnation of blood in the cervical region, causing pain syndromes in the neck area. Sudden movements are accompanied by unpleasant sensations.
Damage to the left-sided aorta leads to abnormal blood pressure. Due to improper blood supply, the body changes, and the disease begins to gradually develop. The clinical picture of damage to the left transverse sinus occurs due to the immature left vertebral artery.
In mild cases, the problem cannot be noticed, only with the help of MRI images. At night there are severe headaches, nausea and even vomiting. There is a risk of having a heart attack or stroke.
It is more dangerous for health when the work of both the left and right arteries is disrupted. This leads to lifelong disability.
General information about hypoplasia
Full blood circulation in all parts of the brain is possible thanks to the Circle of Willis; it is formed from the right and left branches of the spinal arteries.
Under normal conditions, both the right and left vertebral arteries are equally developed. In the area of the subclavian artery towards the cranial cavity they are divided into small vessels.
The term “hypoplasia” in medicine describes underdevelopment of tissues or organs; it can be either a congenital pathology or an acquired one.
Bilateral hypoplasia is much less common than right- or left-sided, although the latter case is already considered quite rare. But since the body’s adaptive capabilities are not limitless, their depletion very quickly leads to the stage of decompensation and the need for surgical intervention.
Causes of pathology
The problem may begin to form in the womb. When planning pregnancy, it is recommended to study all the factors that influence the occurrence of hypoxia. Many factors can provoke hypoplasia of the right transverse sinus. Main reasons:
- bruises and injuries;
- ionization and radiation waves;
- genetic predisposition.
Cases have been recorded when changes appeared without clearly defined reasons. Experts could not explain this phenomenon. In adults, pathology can occur as a result of:
- osteochondrosis in acute or chronic form;
- dislocation of the vertebrae;
- overgrowth of the nape membrane with bone tissue;
- thrombosis;
- a strong blow to the head;
- spinal bruise;
- atherosclerosis.
Age-related changes provoke progression of the disease. Doctors often make incorrect diagnoses, since the disorder is influenced by many factors.
Causes and consequences of the disease
How does hypoplasia occur?
Factors influencing the occurrence of hypoplasia affect the human body even in the mother's womb, however, the same can be said about most diseases and birth defects.
It is believed that the following processes and phenomena may cause hypoplasia:
- Bruises and various injuries to the mother during pregnancy; Abuse of certain medications, alcohol, nicotine, as well as narcotic substances when carrying a child, toxic chemical compounds can also give a similar effect; Infectious diseases of the expectant mother; Genetic predisposition to diseases of the circulatory system;
Hypoplasia of the vertebral arteries does not always develop due to the above situations; these cases only significantly increase the risk of pathologies in the development and functioning of the circulatory system. But sometimes, however, children with congenital hypoplasia are born in the absence of any of the listed reasons. So modern medical luminaries do not yet have a consensus on this matter, although there are many conflicting theories.
What are the risks of the disease in the future?
In some cases, the defect does not make itself felt until a certain period or even throughout life, since hemodynamic disorders are attributed to other diseases or simply poor health, if the symptoms are not particularly pronounced.
The narrowing of the opening of the artery at the point where it enters the bone canal during hypoplasia significantly impedes the flow of blood to the brain tissue. The consequences of hypoplasia, therefore, can be unpredictable, and in this case it is not immediately possible to identify the real cause of multiple dysfunctions. However, some of them do not pose a serious threat to health, but definitely worsen the quality of life. These include increased fatigue, periodic severe headaches, decreased visual acuity and hearing.
How it develops
The disease appears as a result of a disorder in the structure of the aorta. Defects develop during embryonic maturation. Pontocerebellar hypoplasia occurs due to a lack of CDG (carbohydrate glycoprotein). This deviation is clearly visualized.
The pathology refers to an autosomal recessive disease. There is a delay in the child's development and obvious microcephaly.
The cervical vessels are responsible for supplying blood to the brain; they form a dense circle. If the lumen narrows, life-threatening diseases appear.
Even with minor damage, the cerebellum ceases to receive normal nutrition. The child’s fine motor skills suffer and handwriting cannot be developed. If the brain stem is damaged, facial expressions of the entire face are disrupted, and the swallowing process fails.
With the development of microcephaly, the patient loses weight and volume of the head. The development of internal elements changes and defects appear.
The convolutions lag behind in development and are not deep. The visual tubercle does not protrude and looks smaller. The trunk and pyramid of the oblong part are also much smaller, which affects the size of the skull.
During the process of growth, the facial part of the child grows quickly and the brain part of the skull grows more slowly. As a result, the deformation of the head can be seen with the naked eye.
Optic nerve hypoplasia
Description
Optic nerve hypoplasia
is a common congenital polyetiological non-progressive anomaly caused by a decrease in the number of axons of the affected nerve with normal development of supporting tissue.
In China, patients with optic nerve hypoplasia account for 5.9%
of the total number of blind people aged
5
to
15
years.
Pathogenesis.
Until recently, it was believed that optic nerve hypoplasia develops as a result of impaired differentiation of retinal ganglion cells at the embryonic stage of
13-15
mm, which corresponds to
4-6
weeks of gestation. However, this hypothesis did not explain the phenomenon of the frequent combination of optic nerve hypoplasia with brain malformations and was not confirmed by some histological findings. In particular, in patients with optic nerve hypoplasia, amacrine and horizontal cells, which have common precursors with ganglion cells, remain intact.
It is possible that in some cases, optic nerve hypoplasia is the result of the culmination of axonal regression during apoptosis during the period from 16
1st to
31st
weeks of gestation or the outcome of retrograde degeneration during encephaloclastic processes leading to: the formation of brain defects (porencephaly, hydranencephaly, etc.) and causing damage to the pregeniculate visual pathways.
The frequent combination of cerebral hemispheric anomalies with optic nerve hypoplasia suggests that its development is caused by a violation of the mechanisms of regulation of intrauterine migration - both neurons of the cerebral hemispheres and axons of retinal ganglion cells. Optic nerve hypoplasia may be associated with suprasellar tumors, such as teratoma. Probably, in the prenatal period, when certain parts of the optic pathway are compressed by a neoplasm, the process of normal development of the optic nerve is disrupted.
Etiology.
Teratogenic effects can aggravate normal processes occurring in the prenatal period, such as apoptosis, leading to the development of optic nerve hypoplasia. In experiments on mice and in examining children born to mothers at “high” risk, it was shown that taking cocaine or alcohol during pregnancy leads to a significant increase in the incidence of optic nerve hypoplasia in the offspring.
Optic nerve hypoplasia is detected in 50 %
newborns with fetal alcohol syndrome. Risk factors include the young age of mothers, the presence of insulin-dependent diabetes mellitus, smoking and the use of certain pharmaceuticals (phenobarbital, LSD, quinine, depakine, antidepressants) during pregnancy, and prematurity.
Cases of the development of optic nerve hypoplasia in children who have had intrauterine herpetic or cytomegalovirus infection have been described. No regular chromosomal defects were identified in patients with isolated optic nerve hypoplasia. Meanwhile, Y. Hackenbrach et al. (1975) reported on 5 patients with bilateral optic nerve hypoplasia from different generations of the same family, suggesting an autosomal dominant mode of inheritance. A. J. Churchill et al. (2000) found a PAX6 gene mutation at 11p13 in a father and son with ocular changes including aniridia, early-onset cataracts, and optic nerve hypoplasia. Mutation of the PAX6 gene at the 11p13 locus leads to the development of aniridia. It is important to note that the genetic defect in my son was verified prenatally - during amniocentesis at 16
th week of gestation.
Histological studies.
Morphological studies of eyes with optic nerve hypoplasia revealed a decrease in the number of retinal ganglion cells.
Horizontal and amacrine cells appear normal and their number does not decrease. The area surrounding the reduced optic disc is covered by retinal pigment epithelium, which creates an ophthalmoscopic “double ring” effect. A histological study of hypoplastic optic nerves of fetuses extracted from rats on a diet containing 5%
alcohol revealed a significant decrease in the cross-section of the affected nerve.
Electron microscopy revealed changes in the neuropil, a decrease in the number of astroblasts and the presence of pyknotic nuclei in some of them, atrophy and degeneration of optic nerve axons, ultrastructural disorders of the myelin sheaths, astrocytes and oligodendrocytes. Signs of extraisluteal edema were found in the layers of the retina (outer nuclear, nerve fibers and ganglion cells), a decrease in the number and size of axons, axonal and periaxonal edema (between the axolemma and the nerve sheath), and thinning of myelin. K. Sawada et al. (2002) revealed selective loss of small-diameter myelinated axons in rabbits born to dams exposed to ethanol during pregnancy between days 10 and 21 of gestation. Clinical manifestations.
The first clinical description of optic nerve hypoplasia belongs to W. Newman (1864).
Optic nerve hypoplasia varies in severity and can be either unilateral or bilateral. In infants with severe forms of the disease, parents notice strabismus, nystagmus and lack of adequate visual orientation in the child already at the age of 2-3
MSS.
Nystagmus and/or strabismus are detected in 86-92%
of children with optic nerve hypoplasia. With unilateral or asymmetrical lesions, deviation of the eye with more pronounced changes in the disc is noted. Often, patients with optic nerve hypoplasia have an afferent pupillary defect.
Ophthalmoscopic manifestations of optic nerve hypoplasia:
- the “double ring” symptom (Fig. 13.1, 13.2), due to the fact that the optic disc, reduced in diameter and often decolorized, is surrounded by retinal pigment epithelium, filling the space from its edges to the border of the scleral canal, which has normal dimensions;
- lack of differentiation of the polar and foveal reflexes (see Fig. 13.2; Fig. 13.3);
- corkscrew-shaped hodretinal vessels (see Fig. 13.1), having a normal caliber.
Damage to the optic nerve can be isolated, but is often combined with ametropia (myopia, myopic or hypermetropic astigmatism) and other eye anomalies (microphthalmos, congenital cataracts, aniridia, primary persistent hyperplastic vitreous body, etc.).
Optic nerve hypoplasia in systemic lesions.
Brain malformations are detected in
50%
of children with optic nerve hypoplasia.
Cases of a combination of optic nerve hypoplasia with hemispheric migration anomalies (schizencephaly or cortical hegerotopia), as well as with intrauterine and/or perinatal damage of hypoxic-ischemic, toxic-dismetabolic or infectious etiology (periventricular leukomalacia, cortical and subcortical encephalomalacia) have been described. Hemispheric abnormalities in newborns with optic nerve hypoplasia can be considered an unfavorable prognostic criterion indicating future neurological abnormalities. Neurological symptoms are observed in 20%
of patients with optic nerve hypoplasia.
In 1956, G. sk-Morsier described the so-called septo-optic dysplasia, which includes the following triad of symptoms: optic nerve hypoplasia, agenesis or thinning of the corpus callosum and septum pellucidum (Fig. 13.4).
Septo-optic dysplasia is often combined with pituitary insufficiency (which can manifest as severe growth retardation) and neurological disorders (convulsions, paresis, etc.).
Septooptic dysplasia has been found to develop more often in children born to mothers aged 20
years or younger.
Endocrine dysfunctions are determined in 27—43 %
children with optic nerve hypoplasia.
Posterior pituitary ectopia, which is essentially a pathognomonic sign of anterior pituitary hormone deficiency, is detected on MRI in approximately 15%
of patients with optic nerve hypoplasia. Growth hormone deficiency is the most common endocrine disorder associated with optic nerve hypoplasia. Other endocrine disorders are less common: hypothyroidism, panhypopituitarism, diabetes insipidus, hyperprolactinemia.
Optic nerve hypoplasia is a symptom that is important for the differential diagnosis of certain malformations: Patau syndromes (trisomy 13
th chromosome), Alert, Warburg, Meckel-Gruber, Zellweger's disease or cerebrohepatorenal syndrome. Detection of optic nerve hypoplasia in patients with Warburg syndrome (autosomal recessive oculocerebral syndrome) is a diagnostic “key” to distinguish this disease from common defects of neural tube development.
Cases of unilateral and bilateral optic nerve hypoplasia have been described in children with frontonasal dysplasia and basal encephalocele, as well as in patients with epidermal nevus of Jadassohn. DAThompson et al. (1999) reported a patient with bilateral optic nerve hypoplasia, achiasmia, cleft lip and hard palate, nasosphenovdal encephalocele, agenesis of the corpus callosum, and absence of the falx cerebri. The combination of bilateral optic nerve hypoplasia with achiasmia, absence of optic tracts and focal polymicrogyria of the left perisylvian region in a 5-month-old infant was described by K. Waheed et al. (2002).
Optic nerve hypoplasia occurs in 30—57
% of patients with Eicardi syndrome, which is characterized by agenesis of the corpus callosum, myoclonic seizures, mental retardation and lacunar chorioretinal lesions.
Visual functions. Visual acuity with optic nerve hypoplasia varies from 1,0
to “absence of veto sensation.”
Visual field impairments detected in patients with optic nerve hypoplasia are quite diverse: local central and/or peripheral loss, hemianoptic defects, concentric narrowing.
Inferonasal and inferior colliculose defects in the visual field have been described in children with superior segmental optic nerve hypoplasia born to mothers with insulin-dependent diabetes mellitus. Optical coherence tomography in these patients reveals segmental thinning of the retinal nerve fiber layer and (in some cases) abnormal enlargement of the retinal pigment epithelium-choroid complex over the edge of the lamina cribrosa.
The polymorphism of visual field defects is explained by the variety of morphological disorders in patients with optic nerve hypoplasia. Not only the disc and optic nerve can be hypoplastic, but also the chiasm, optic tract, and any segment of the retrogenic visual pathways, such as optic radiation. Hypoplasia of optic radiation was described by M. Brodsky et al. (1997) in a child with congenital peripapillary staphyloma, atypical hemimegacephaly and seborrheic nevus of Jadassohn. In patients with combined hypoplasia of the optic nerve, chiasm and/or retrogenic visual pathways, hemianoptic or quadrantoptic visual field defects are determined. As a rule, color vision disorders are not detected.
Electrophysiological studies.
ERG with optic nerve hypoplasia usually remains normal, while G.Cibis and K.Fitzgerald (1994) found a decrease in ERG amplitude in
42%
of patients with optic nerve hypoplasia. The authors explained the detected changes by transsynaptic degeneration of structures lying distal to ganglion cells, which, however, was not convincingly argued.
The electrooculogram does not change in isolated optic nerve hypoplasia. The most informative test for assessing visual functions in children with optic nerve hypoplasia is recording of visual evoked potentials (VEP). The amplitude and latency values of the main positive component P100 of VEP in optic nerve hypoplasia correlate with the size of the optic nerve head. This dependence is likely due to the number of neurons involved in generating responses. With a diameter of the optic nerve head from 0,1
up to
0.25
RD (see Fig. 13.1) VEP, as a rule, are not recorded (Fig. 13.5). Visual acuity in these children usually fluctuates within the range of “O—correct light projection.”
In cases where the disk diameter is 0.3–0.5 RD (see Fig. 13.2), VEPs are recorded in response to a flash stimulus or reversal patterns with cell sizes of 220–55′
.
The latency of a P100 VEP is significantly increased, and the amplitude is reduced compared to the age norm (see Fig. 13.5). Visual acuity in these children varies from 0.005
to
0.04
.
In patients whose disc diameter exceeds 0.6 RD (see Fig. 13.3), they are recorded for a flash stimulus and for patterns with cell sizes of 110-7′ (Fig. 13.6). In these patients, the latency is increased and the amplitude of the RJO pattern-VEP component is decreased, and visual acuity is 0.03-1.0
. The VEP recording method is useful for determining the severity of visual impairment and predicting functional outcomes in young children with optic nerve hypoplasia.
X-ray studies.
In patients with optic nerve hypoplasia, a decrease in the size of the optic canal is often determined by canal x-ray or axial x-ray tomography, but no direct correlation has been found between the severity of optic nerve damage and canal diameter.
This is not surprising, since even in healthy people there can be a difference in the parameters of the visual channel between the right and left orbits, sometimes reaching 20%
. Currently, the use of routine radiological methods for diagnostic purposes in patients with suspected optic nerve hypoplasia has lost its relevance due to the widespread introduction of X-ray computed tomography, magnetic resonance imaging and neurosonography (NSG) into clinical practice.
Neuroradiological and ultrasound studies.
CT scan of the orbit and brain in some cases can reveal thinning of the optic nerve in its orbital part (Fig. 13.7), as well as a decrease in the diameter of the optic opening of the orbit, hemispheric migration anomalies, periventricular leukomalacia, encephalomalacia, anomalies of midline brain structures (underdevelopment or agenesis corpus callosum, absence of a transparent septum), etc.
NSG has comparable resolution capabilities to CT and MRI when examining the brain in children under 1 year of age. NSG allows us to identify changes in the central nervous system combined with optic nerve hypoplasia, in particular malformations of the brain [holoprosenyphaly (Fig. 13.8),
agenesis of the corpus callosum and septum pellucidum (see Fig. 13.4), agyria, schizencephaly (Fig. 13.9),
hydranencephaly (Fig. 13.10), etc.]
and pathology caused by hypoxic-ischemic disorders [periventricular leukomalacia (Fig. 13.11), etc.], hemorrhagic and inflammatory intracranial lesions in young children with an open anterior fontanel. NSG has a number of advantages over CG and MRI: the duration of the study, the need for contrast, the absence of image disintegration during movement (anesthesia is not required), the absence of exposure to ionizing radiation, portability and the relative cheapness of the equipment.
NSG is indicated for all infants with optic nerve hypoplasia, and children with hypoglycemia, especially those born to mothers under the age of 20 years, should undergo MRI to exclude possible neuroendocrine dysfunctions.
MRI is an optimal non-invasive diagnostic method in terms of its resolution capabilities, as it allows not only to establish the correct diagnosis in controversial cases, but also to carry out a rather complex differential diagnosis with various neuroendocrine diseases, often combined with optic nerve hypoplasia.
When using coronal and sagittal sections, it is possible to identify a decrease in the diameter of the intraorbital and intracranial parts of the optic nerve, diffuse thinning or absence of the chiasm in a bilateral process (chiasmatic hypoplasia or achiasmia), hypoplasia of the optic tract, hypoplasia or posterior ectopia of the pituitary gland, and abnormalities of the structures of the midline of the brain.
Detection of infundibular hypoplasia or posterior ectopia of the pituitary gland in children with optic nerve hypoplasia during MRI is a prognostic criterion indicating the development of endocrine insufficiency in the future.
According to neuroradiological studies, agenesis of the corpus callosum and/or septum pellucidum is determined in 46—53 %
patients with optic nerve hypoplasia, other malformations of the central nervous system - in
12-45%
of cases.
Differential diagnosis.
Despite the characteristic ophthalmoscopic picture, the correct diagnosis in patients with optic nerve hypoplasia is often established only at an older age. Problems with ophthalmoscopic diagnosis in infants are associated, as a rule, with their active behavior during examination. Patients are often observed for a long time with the diagnosis of “optic nerve atrophy” Differential diagnosis Hypoplasia and atrophy of the optic nerve usually causes difficulties in bilateral lesions and is based only on ophthalmoscopy data. In patients with optic nerve hypoplasia, the disc may have a white or gray color, but it is always reduced in size. Additional signs in favor of optic nerve hypoplasia are the “double ring” symptom and corkscrew tortuosity of the vessels.
Difficulties in interpreting the ophthalmoscopic picture may arise when examining patients with high hyperopia, when the examination creates the false impression that the optic disc has a smaller diameter.
In difficult cases, auxiliary diagnostic methods are used:
- calculation of the ratio of the disc-macula distance to the disc diameter (normally <3) during conventional photographic recording;
- measuring disk parameters using a computer disk analyzer;
- photographing the fundus in red-free light with high resolution, allowing to determine the defect of the nerve fiber layer;
- study of the thickness of the nerve fiber layer using optical coherence tomography, which is especially informative if there are corresponding changes in the field of view.
Hypoplasia of the optic nerve must be differentiated from its aplasia. These conditions have clear clinical differences: with optic seal hypoplasia, even if the optic seal disc is practically indistinguishable, the central retinal vessels are always identified, having a normal caliber and a corkscrew-like course.
If optic nerve hypoplasia is detected in a young child, the ophthalmologist should rule out possible subclinical endocrine or neurological disorders as quickly as possible.
A thorough examination using immunobiochemical and neuroradiological methods will make it possible to diagnose neuroendocrine disorders even before the clinical manifestation of the disease and prescribe adequate therapy to the child, which will prevent the development of irreversible complications. In these situations, the use of MRI helps to obtain information necessary for differential diagnosis and neurosomatic prognosis. A history of neonatal jaundice in infants with optic nerve hypoplasia suggests secondary hypothyroidism, while neonatal hypoglycemia or paroxysms indicate panhypopituitarism. Therefore, MRI should be used to rule out secondary neonatal hypothyroidism in infants with optic nerve hypoplasia. Due to the obvious difficulty in assessing normal growth hormone levels, most patients with optic nerve hypoplasia should be monitored by a pediatrician. In case of growth retardation, biochemical studies are necessary to confirm the diagnosis. Children with optic nerve hypoplasia, hypoglycemia, neonatal jaundice, and posterior pituitary ectopia on MRI usually have anterior pituitary hormonal deficiency. Such patients are indicated for a detailed endocrinological examination.
Agenesis of the septum pellucidum and/or corpus callosum detected by NSG, CT or MRI is not a reliable sign of neurological disorders or hormonal deficiency. It is possible to predict the appearance of neurological abnormalities in infants with optic nerve hypoplasia and hypo- or agenesis of the corpus callosum or septum pellucida only when these malformations are combined with hemispheric migration anomalies.
Treatment.
Some authors are skeptical about the treatment of patients with optic nerve hypoplasia.
Our experience shows that rehabilitation attempts made at an early age in children with optic nerve hypoplasia, in some cases, lead to positive results. In addition, some development of visual functions in children of the first year of life with optic nerve hypoplasia may be due to the ongoing maturation of pre- and postgenic visual pathways and cortical centers. It is known that by 6
months of age the volume of the human lateral geniculate body increases by
2
times, and until
the 4th
month in the postnatal period the number of spines on the dendrites and soma of neurons increases.
Synaptogenesis in
Brodmann's
17 the 8th
month of life, and at the same time there is an increase in the width of the cortex in all visual fields. The described processes, characteristic of healthy infants, also occur in children with optic nerve hypoplasia. Due to the plasticity of the nervous system in young children, treatment carried out during this period allows achieving better functional results.
Rehabilitation of children with optic nerve hypoplasia primarily involves eliminating the fatal effect of visual deprivation on the maturing visual system. In this regard, functional rehabilitation of children with optic nerve hypoplasia primarily includes measures to prevent the development of amblyopia (refractive, dysbinocular, etc.) and its treatment. Children with optic nerve hypoplasia should be prescribed spectacle or contact correction of ametropia, dosed occlusion of the better-seeing eye in case of unilateral or asymmetrical lesions, laser pleoptics and transcutaneous electrical stimulation of the optic nerve as early as possible. Surgical treatment of strabismus is possible for a cosmetic or, in the presence of high visual acuity, a functional purpose (development of binocular vision). At the same time, it is necessary to correct somatic and neuroendocrine disorders.
Article from the book: Visual functions and their correction in children | S.E. Avetisov, T.P. Kashchenko, A.M. Shamshinova.
Symptoms
Signs of hypoplasia are observed in older age, such as migraines, high blood pressure, drowsiness, psychological disorders and decreased sensitivity. The blood supply gradually returns to normal on its own. In such cases, the patient consults a doctor.
Possible symptoms:
- dizziness and pain;
- nerve dysfunction;
- disturbances of sensitivity, speech and motor apparatus;
- hallucinations.
Diagnostics
In order for the doctor to make a correct diagnosis, neurological examinations should be completed, the results of which determine the presence of the disease. The patient will need to consult a neurologist, therapist, cardiologist and ophthalmologist.
The doctor prescribes an MRI, then ultrasound scanning, Dopplerography and angiography. All methods will help determine the shape, size and structure of blood vessels. Experts also look at the state of the circulatory system.
The diagnosis is confirmed if:
- the internal lumen in diameter is less than 2 mm;
- blood flow moves at a speed of less than 40 ml/min;
- there is no stenosis.
As a result of the examination, patients observed:
- stroke anomaly in approximately 9% of cases;
- stenosis – 20%;
- deformation – 40%;
- extravascular compression 10%;
- blockage at 4%.
Basically, damage to the blood supply to the left side is diagnosed when a person experiences cardiovascular failure. Even if you regularly visit a specialist, it is impossible to notice the disorder.
Damage to the left side appears in adults, and symptoms should be closely monitored. The first sign is pain in the neck. Other symptoms may be absent and making an accurate diagnosis difficult.
About 25% of people have a disorder in the circulatory system, half of them can live their whole lives and not know about it. The initial stage of the pathology does not cause serious manifestations.
Who's at risk
People whose relatives have been diagnosed with cerebral hypoplasia are most susceptible to the disease. Children are at particular risk; the disease can appear in the womb.
In a relative risk group, people over 35 years of age have reduced immune defenses and are at risk of developing the disorder. In a man over 40 years of age, sperm may be genetically affected; a woman's body over 35 years of age does not recognize the changes.
Children with the disease may be born to a mother with chronic thyroid disorders or to a diabetic.
Cerebellar hypoplasia - symptoms and treatment
Symptoms of cerebellar hypoplasia are:
- Delayed physical and mental development. Children start sitting and walking late. Their speech development is delayed;
- Tremor (shaking) of the head and limbs, which often appears in the first year of life;
- Difficulty maintaining balance;
- Clumsy gait. Often, children with cerebellar hypoplasia can only move with assistance.
With age, the symptoms of the disease stabilize. And usually after 10 years of age, no further growth is observed. In some patients, cerebellar hypoplasia leads to the development of mental deficiency, accompanied by deafness and/or blindness. Unfortunately, medicine is currently unable to cure children with this disease. However, long-term social and motor rehabilitation - for example, classes with a speech therapist, occupational therapy, balance therapy, massage, physical therapy - can improve the condition of patients and instill in them self-care skills.
Even if a child has been given such a terrible diagnosis, there is no point in giving up. Such a baby, much more than other children, needs affection, attention and care - and mother’s love sometimes works wonders!
Treatment methods
Treatment may be necessary if severe deformation occurs, patency is impaired and blood flow begins to flow poorly. The patient requires surgical treatment if there is a threat of ischemic stroke.
Drug treatment
Glycine tablets Phezam (capsules) Mexidol injections
Complex therapy is prescribed with the help of pharmacological drugs. They help blood circulation function normally, strengthen the walls of blood vessels, and stimulate metabolism in cells. Medicines prescribed by a specialist:
- nootropics;
- blood flow regulators;
- metabolism accelerators;
- vasodilators.
If the tissues are affected, the patient has severe hypoxia, then the following drugs are suitable: Actovegin, Reomacrodex.
Surgery
Surgery is necessary for people whose large aortas are damaged. The size and type of deformity is diagnosed, and then specialists decide whether surgery is necessary. The decision is influenced by the patient’s well-being and possible risks.
During the operation, deficiencies are corrected. After about 3 months, it is necessary to check the operated area. Doctors do this using the instrumental method.
Folk remedies
Recipes for folk remedies are used for symptomatic treatment at the initial stage of pathology.
In severe cases, traditional medicine methods do not bring the desired effect. For additional therapy that will improve immunity, you can prescribe decoctions or tinctures, lemon, olive oil and chamomile flowers help. Folk remedies have sedative, antihypertensive and vasodilating properties. Suitable:
- infusions and decoctions of rose hips and hawthorn;
- lemon balm and mint teas;
- extract of valerian, motherwort.
Treatment method for hypoplasia
Paradoxically, in certain cases a person does not need treatment for hypoplasia of the vertebral artery, since the body’s adaptive capabilities allow it to cope with hemodynamic disturbances for a long time and prevent the appearance of clinical symptoms in principle, and the blood supply to the brain does not deteriorate.
But if signs of the disease have already manifested themselves, then you should not delay visiting a doctor, since vivid symptoms almost always indicate quite serious health problems. Most often this occurs due to atherosclerosis, with constant high physical and emotional stress, as well as with malfunctions of compensatory mechanisms.
Atherosclerosis, as well as vascular stenoses of a different nature, is one of the main causes of hypoplasia. Therefore, in order to get rid of health problems, treatment must be comprehensive and exclude phenomena that painfully constrict blood vessels.
In this case, treatment of hypoplasia should be started as soon as possible in order to prevent a significant deterioration in well-being and, if possible, to avoid surgery, although most often surgery (for example, stenting and/or angioplasty) is the only alternative, since the disease becomes severe.
With a relatively early diagnosis, specialists still try to avoid surgical intervention in the patient’s body with the help of drug therapy. Drugs that dilate blood vessels and lower blood pressure are the basis of treatment, and nootropics are recommended as an adjuvant.
In addition to the above methods, modern medicine does not have any other means, although some “alternative medicine centers” offer other procedures as therapy - acupuncture, massage, various gymnastic complexes. You should not unconditionally rely on the promises of people who most often do not even have a special education. If desired, and only after consultation with your doctor, you can combine both methods.