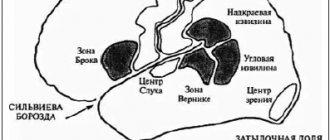
Autoregulation of cerebral blood flow (BFF) is a protective mechanism aimed at maintaining cerebral blood flow in response to changes in cerebral perfusion pressure (CPP) and cerebrovascular resistance (CVR) [1]. The term was proposed by N. Lassen in 1959 [2]. In this case, CPP is the difference between mean arterial pressure and intracranial pressure (ICP) and is inversely proportional to CVR. CVR, in turn, represents the total vascular resistance, including arterioles of the pia mater and penetrating precapillary arterioles of the brain [3]. The speed of cerebral blood flow is directly proportional to CPP and inversely proportional to CVR.
There are a number of mechanisms involved in the regulation of maintaining the level of cerebral blood flow (the norm is 50 ml/100 g/min): 1) metabolic
(blood pH, balance of CO2/O2 dissolved in the blood, nitric oxide, adenosine, products of the functioning of astrocytes and neurons) [4, 5];
2) myogenic
(Ostroumov-Beilis effect - a reaction from the smooth muscle layer of arteries in the form of contraction when blood pressure increases and relaxation when blood pressure decreases);
3) peripheral (or systemic) (activity of the sympathoadrenal system, carotid glomeruli, temperature, endothelial factors); 4) neurogenic
(vasomotor center, centers for regulating the activity of the sympathetic system and, possibly, a number of other brain structures). The links of the latter are the least studied.
All AUB mechanisms ensure the maintenance of cerebral perfusion when blood pressure fluctuates between 60-70 and 170-180 mmHg. When blood pressure increases or decreases beyond this range, a breakdown of autoregulation occurs—a condition in which cerebral blood flow passively depends on systemic blood pressure, and ICP becomes directly dependent on blood pressure. This condition is dangerous both due to the development of ischemia and the development of “luxury perfusion syndrome”, reactive hyperemia, associated with a high risk of secondary ischemic or hemorrhagic complications.
BUN is often disrupted in the acute period of severe traumatic brain injury (sTBI), which occurs with hemodynamic instability and is associated with an unfavorable outcome. Assessing the state of the AUB currently serves as the main objective guideline for monitoring and correcting conservative treatment and making a decision on neurosurgical intervention in the acute period of mTBI [6]. For patients with impaired autoregulation due to TBI, achieving the recommended level of CPP (not lower than 70 mm Hg) may be associated with cerebral hyperemia, predisposing to the formation of intracranial hypertension, edema and intracerebral hemorrhage [6]. Therefore, according to the latest recommendations, for this group of patients the required CPP limit is reduced to 60 mmHg. [7]. Recent studies [3] have shown that maintaining CPP levels below 50-60 mm Hg. is associated with a large number of positive outcomes, while CPP is more than 70-80 mm Hg. more often leads to unfavorable outcomes in patients with impaired BUN.
In the classic works of N. Lundberg [8], three types of spontaneous ICP oscillations are identified: A-waves (plateau), B- and C-waves. Subsequent studies showed that plateau waves reflect cerebral vasodilation, which leads to an increase in cerebral blood flow and, as a consequence, an increase in ICP [9]. Currently, one of the most reliable and safe methods for continuous assessment of the autoregulation of the state of cerebral vessels in the acute period of mTBI is based on the analysis of wave fluctuations of arterial and intracranial pressure - monitoring the reactivity coefficient of cerebral vessels
(pressure reactivity index -
P R x
). PRx is the correlation coefficient between slow-wave fluctuations in blood pressure and ICP [10, 11]. It has been shown that plateau waves of ICP in patients with TBI are more often recorded when the autoregulation of cerebral vessels is preserved. At the moment of formation of plateau waves, maximum vasodilation of arterioles develops and autoregulation is lost, which is recorded by an increase in PRx [12].
Previously, experimental studies [13, 14] showed that damage to certain structures of the brain stem and hypothalamus is accompanied by the development of cerebral edema. Electrical stimulation of individual brainstem structures initiates processes leading to cerebral vasodilation and an increase in volumetric cerebral blood flow [15, 16]. It is assumed that the brainstem may be directly involved in the generation of waves characteristic of intracranial hypertension, but the anatomical pathways and mechanisms of this influence are still not well understood. A number of experimental studies have shown the effect of stimulation of individual structures of the brainstem (including the locus coeruleus - locus coeruleus) of the reticular formation of the medulla oblongata and hemispheric structures of the brain (in particular, the anterior cingulate gyrus and anterior parts of the hypothalamus) on changes in ICP [17]. It was also previously shown that damage to brain stem structures and frontal lobes during TBI can lead to changes in the activity of the sympathetic nervous system, affecting arterial tone [18].
A feature of DAP is the predominant damage to the subcortical and stem structures of the brain involved in maintaining vital functions, including the regulation of cerebral vascular tone and BUN.
The purpose of this work is to identify anatomical damage to deep brain structures that influence the degree and duration of BUN disturbances in the acute period of severe DAP.
General concept
In the process of changes in the functional activity of each organ and tissue, as well as their metabolic needs, regulation of blood circulation occurs. The physiology of the human body is such that this process is carried out in three main directions.
The first way to adapt to changing conditions is regulation through the vascular system. To measure this indicator, the amount of blood in a certain time period is determined. This could be a minute, for example. This indicator is called minute blood volume (MBV). This amount is capable of meeting the needs of tissues during metabolic reactions.
The second direction of ensuring regulation processes is maintaining the required pressure in the aorta, as well as other large arteries. This is the driving force that ensures sufficient blood flow at any given moment. Moreover, it must move at a certain speed.
The third direction is the volume of blood, which is determined in the systemic vessels at a given time. It is distributed among all organs and tissues. At the same time, their need for blood is determined. To do this, their activity and functional loads at the moment are taken into account. During such periods, the metabolic needs of tissues increase.
Regulation of blood circulation occurs under the influence of these three processes. They are inextricably linked. In accordance with this, the regulation of the heart, local and systemic blood flow occurs.
To calculate the IOC, you will need to determine the amount of blood that the left or right cardiac ventricle ejects into the vascular system per minute. Normally, this figure is about 5-6 l/minute. Age-related features of blood circulation regulation are compared with other norms.
Mechanisms of regulation of cerebral circulation and compensation for its disorders
Regulation of cerebral circulation is carried out by a complex system, including intra- and extracerebral mechanisms. This system is capable of self-regulation (i.e., it can maintain blood supply to the brain in accordance with its functional and metabolic needs and thereby maintain a constant internal environment), which is carried out by changing the lumen of the cerebral arteries. These homeostatic mechanisms, developed in the process of evolution, are very sophisticated and reliable. Among them, the following main mechanisms of self-regulation are distinguished.
Nervous mechanism
transmits information about the state of the object of regulation through specialized receptors located in the walls of blood vessels and tissues. These, in particular, include mechanoreceptors localized in the circulatory system, reporting changes in intravascular pressure (baro- and pressoreceptors), including pressoreceptors of the carotid sinus; when irritated, cerebral vessels dilate; mechanoreceptors of the veins and meninges, which signal the degree of their stretching with an increase in blood supply or brain volume; chemoreceptors of the carotid sinus (when irritated, the cerebral vessels narrow) and the brain tissue itself, from where information comes about the content of oxygen, carbon dioxide, pH fluctuations and other chemical shifts in the environment during the accumulation of metabolic products or biologically active substances, as well as receptors of the vestibular apparatus, aortic reflexogenic zone, reflexogenic zones of the heart and coronary vessels, a number of proprioceptors. The role of the sinocarotid zone is especially important. It affects cerebral circulation not only indirectly (through total blood pressure), as previously thought, but also directly. Denervation and novocainization of this zone in the experiment, eliminating vasoconstrictor effects, leads to dilation of cerebral vessels, increased blood supply to the brain, and an increase in oxygen tension in it.
Humoral mechanism
consists in the direct impact on the walls of effector vessels of humoral factors (oxygen, carbon dioxide, acidic metabolic products, K ions, etc.) through the diffusion of physiologically active substances into the vascular wall. Thus, cerebral circulation increases with a decrease in oxygen content and (or) an increase in carbon dioxide content in the blood and, conversely, weakens when the content of gases in the blood changes in the opposite direction. In this case, reflex dilatation or constriction of blood vessels occurs as a result of irritation of the chemoreceptors of the corresponding arteries of the brain when the content of oxygen and carbon dioxide in the blood changes. An axon reflex mechanism is also possible.
Myogenic mechanism
implemented at the level of effector vessels. When they are stretched, the tone of the smooth muscles increases, and when they contract, on the contrary, it decreases. Myogenic reactions can contribute to changes in vascular tone in a certain direction.
Different regulatory mechanisms do not act in isolation, but in various combinations with each other. The regulatory system maintains constant blood flow in the brain at a sufficient level and quickly changes it when exposed to various “disturbing” factors.
Thus, the concept of “vascular mechanisms” includes the structural and functional characteristics of the corresponding arteries or their segments (localization in the microcirculatory system, caliber, wall structure, reactions to various influences), as well as their functional behavior - specific participation in certain types of regulation of peripheral blood circulation and microcirculation.
Clarification of the structural and functional organization of the vascular system of the brain made it possible to formulate a concept about the internal (autonomous) mechanisms of regulation of cerebral circulation under various disturbing influences. According to this concept, in particular, the following were identified: the “closing mechanism” of the main arteries, the mechanism of the pial arteries, the mechanism for regulating the outflow of blood from the venous sinuses of the brain, the mechanism of intracerebral arteries. The essence of their functioning is as follows.
The “closing” mechanism of the main arteries maintains constant blood flow in the brain during changes in the level of total blood pressure. This is accomplished through active changes in the lumen of the cerebral vessels - their narrowing, which increases resistance to blood flow when total blood pressure increases, and, conversely, expansion, which reduces cerebrovascular resistance when total blood pressure falls. Both constrictor and dilator reactions arise reflexively from extracranial pressoreceptors, or from receptors in the brain itself. The main effectors in such cases are the internal carotid and vertebral arteries. Thanks to active changes in the tone of the main arteries, respiratory fluctuations in total arterial pressure, as well as Traube-Hering waves, are damped, and then the blood flow in the vessels of the brain remains uniform. If changes in total blood pressure are very significant or the mechanism of the main arteries is imperfect, as a result of which adequate blood supply to the brain is disrupted, then the second stage of self-regulation begins - the mechanism of the pial arteries is activated, reacting similarly to the mechanism of the main arteries. This whole process is multi-part. The main role in it is played by the neurogenic mechanism, but the peculiarities of the functioning of the smooth muscle membrane of the artery (myogenic mechanism), as well as the sensitivity of the latter to various biologically active substances (humoral mechanism) are also of certain importance.
With venous stagnation caused by occlusion of large cervical veins, excess blood supply to the vessels of the brain is eliminated by weakening the blood flow into its vascular system due to constriction of the entire system of main arteries. In such cases, regulation also occurs reflexively. Reflexes are sent from mechanoreceptors of the venous system, small arteries and meninges (veno-vasal reflex).
The system of intracerebral arteries is a reflexogenic zone, which, under pathological conditions, duplicates the role of the sinocarotid reflexogenic zone.
Thus, according to the developed concept, there are mechanisms that limit the influence of total blood pressure on cerebral blood flow, the correlation between which largely depends on the intervention of self-regulatory mechanisms that maintain the constancy of cerebral vascular resistance (Table 1). However, self-regulation is possible only within certain limits, limited by the critical values of the factors that are its triggers (the level of systemic blood pressure, oxygen tension, carbon dioxide, as well as the pH of the brain substance, etc.). In a clinical setting, it is important to determine the role of the initial blood pressure level, its range within which cerebral blood flow remains stable. The ratio of the range of these changes to the initial pressure level (an indicator of self-regulation of cerebral blood flow) in
to a certain extent, determines the potential possibilities of self-regulation (high or low level of self-regulation).
Disturbances in self-regulation of cerebral circulation occur in the following cases.
1. With a sharp decrease in total blood pressure, when the pressure gradient in the circulatory system of the brain decreases so much that it cannot provide sufficient blood flow to the brain (at a systolic pressure level below 80 mm Hg). The minimum critical level of systemic blood pressure is 60 mm Hg. Art. (at baseline – 120 mm Hg). When it falls, cerebral blood flow passively follows the change in total blood pressure.
2. In case of an acute significant increase in systemic pressure (above 180 mm Hg), when myogenic regulation is disrupted, since the muscular apparatus of the cerebral arteries loses the ability to withstand the increase in intravascular pressure, as a result of which the arteries dilate, cerebral blood flow increases, which is fraught with “mobilization” » blood clots and embolism. Subsequently, the walls of blood vessels change, and this leads to cerebral edema and a sharp weakening of cerebral blood flow, despite the fact that systemic pressure continues to remain at a high level.
3. With insufficient metabolic control of cerebral blood flow. Thus, sometimes after restoration of blood flow in the ischemic area of the brain, the concentration of carbon dioxide decreases, but the pH remains at a low level due to metabolic acidosis. As a result, the vessels remain dilated and cerebral blood flow remains high; oxygen is not fully utilized and the flowing venous blood is red (overperfusion syndrome).
4. With a significant decrease in the intensity of blood oxygen saturation or an increase in carbon dioxide tension in the brain. At the same time, the activity of cerebral blood flow also changes following changes in systemic blood pressure.
When self-regulation mechanisms fail, the arteries of the brain lose their ability to narrow in response to increased intravascular pressure and passively expand, as a result of which excess blood under high pressure is directed into small arteries, capillaries, and veins. As a result, the permeability of the vascular walls increases, protein leakage begins, hypoxia develops, and cerebral edema occurs.
Thus, cerebral circulatory disorders are compensated to certain limits due to local regulatory mechanisms. Subsequently, general hemodynamics is also involved in the process. However, even in terminal conditions, for several minutes, due to the autonomy of cerebral circulation, blood flow is maintained in the brain, and oxygen tension drops more slowly than in other organs, since nerve cells are able to absorb oxygen at such a low partial pressure in the blood, at which other organs and tissues cannot absorb it. As the process develops and deepens, the relationship between cerebral blood flow and systemic circulation is increasingly disrupted, the reserve of autoregulatory mechanisms runs out, and blood flow in the brain increasingly begins to depend on the level of total blood pressure.
Thus, compensation for cerebral circulatory disorders is carried out using the same regulatory mechanisms functioning under normal conditions, but more intense.
Compensation mechanisms are characterized by duality: compensation of some disorders causes other circulatory disorders, for example, when blood flow is restored in a tissue that has experienced a shortage of blood supply, it can develop post-ischemic hyperemia in the form of excess perfusion, contributing to the development of post-ischemic cerebral edema.
The ultimate functional task of the cerebral circulatory system is adequate metabolic support for the activity of cellular elements of the brain and timely removal of the products of their metabolism, i.e. processes occurring in the microvessel-cell space. All reactions of cerebral vessels are subordinated to these main tasks. Microcirculation in the brain has an important feature: in accordance with the specifics of its functioning, the activity of individual areas of the tissue changes almost independently of other areas of it, therefore microcirculation also changes mosaically - depending on the nature of the functioning of the brain at one time or another. Thanks to autoregulation, the perfusion pressure of the microcirculatory systems of any part of the brain is less dependent on the central circulation in other organs. In the brain, microcirculation increases with an increase in metabolic rate and vice versa. The same mechanisms also function in pathological conditions, when there is inadequate blood supply to the tissue. Under physiological and pathological conditions, the intensity of blood flow in the microcirculatory system depends on the size of the lumen of the vessels and on the rheological properties of the blood. However, regulation of microcirculation is carried out mainly through active changes in the width of blood vessels, while at the same time, in pathology, changes in blood fluidity in microvessels also play an important role.
Blood movement
Regulation of cerebral circulation, as well as all organs and tissues of the body, occurs through the movement of blood through the vessels. Veins, arteries and capillaries have a certain diameter and length. They practically do not change under the influence of various factors. Therefore, regulation of blood movement occurs by changing its speed. It moves thanks to the work of the heart. This organ creates a pressure difference between the beginning and end of the vascular bed. Like all fluids, blood moves from an area of high to an area of low pressure. These extreme points are located in certain areas of the body. The highest pressure is determined in the aorta and pulmonary arteries. As blood passes through the body, it returns back to the heart. The lowest pressure is determined in the hollow (lower, upper) and pulmonary veins.
The pressure drops gradually, as a lot of energy is spent pushing blood through the capillary ducts. Also, the blood flow experiences resistance during movement. It is determined by the diameter of the lumen of the blood vessels, as well as the viscosity of the blood itself. The movement becomes possible due to several other reasons. Among them the main ones are:
- there are valves in the veins that prevent the reverse movement of fluid;
- different pressure in the vessels at the starting and ending points;
- the existence of suction force during inhalation;
- movement of skeletal muscles.
The mechanisms of blood circulation regulation are usually divided into local and central. In the first case, this process occurs in organs and local tissues. In this case, it is taken into account how the organ or department is loaded, how much oxygen it requires for proper functioning. Central regulation is carried out under the influence of general adaptive reactions.
Discussion
Our proposed hypothesis about the influence of certain neurotransmitter structures of the brain as central links on AMB in severe brain injury has not previously been covered in the literature. The data we obtained on the DAP model can be explained by the previously accumulated results of studying other human brain diseases (mainly neurodegenerative) and previous experimental work.
It has now been proven that many neurotransmitter systems can influence blood flow through receptors located on capillaries or perivascular glia. Such effects, in particular, are shown for dopamine, which has two types of receptors: D1- and D2-like receptors, the first of which have vasorelaxing effects, and the second - vasoconstrictor effects. Dopamine in humans has a constricting effect on large cerebral arteries and thereby increases the linear velocity of cerebral blood flow [26].
The substantia nigra functionally belongs to the extrapyramidal system, since it is involved in the regulation of muscle tone while ensuring motor functions. The least known and studied are the anatomical pathways through which it affects autonomic functions: breathing, cardiac activity and vascular tone. The substantia nigra contains two types of neurons, one of which uses dopamine ( pars compacta
), and others (
pars reticulata
) - glutamate.
A number of experimental studies have shown that electrical stimulation pars compacta
causes tachycardia and increased blood pressure [25, 27-29]. Such data indicate that dopaminergic neurons of the substantia nigra activate the central pathway of the cardiovascular depressor center, through which inhibition of sympathetic fibers occurs, causing constriction of the arteries and increased heart rate.
Dopaminergic neurons in the substantia nigra send projections to the basal forebrain system, called the extended amygdala. The “extended amygdala” is closely associated with forebrain and brainstem structures involved in the regulation of the cardiovascular system [29, 30]. Stimulation of its structures, as well as stimulation of the substantia nigra, leads to the suppression of cardiovascular reactions [31, 32], which makes it possible to combine them into a single regulatory system. Previous studies have shown that the activity of dopaminergic neurons in the substantia nigra can be regulated by arterial baroreceptors [33, 34]. Denervation of baroreceptors leads to decreased production and release of dopamine into the striatum. These data point to the important fact that dopaminergic neurons of the substantia nigra may be part of a long central baroreceptor reflex pathway that regulates blood pressure [35].
There is evidence that cholinergic neurons are also involved in the regulation of regional cerebral blood flow [36, 37], and this regulatory mechanism does not depend on regional metabolism and systemic blood pressure. Activation of cholinergic fibers of the Meynert nucleus and septal complex leads to the release of acetylcholine in the cortex and hippocampus, which provokes an increase in cerebral blood flow in these structures. A diffuse increase in blood flow in the cortex during walking is associated with excitation of the vasodilatory system of the basal nucleus of Meynert [36]. Activation of cholinergic neurons in the basal forebrain may contribute to increased ICP and the formation of plateau waves due to vasodilation [17].
It is known that in a number of neurodegenerative diseases of the brain (Parkinson’s disease, multiple system atrophy), autoregulation disorders are also observed, which is associated with autonomic dysfunction [38]. However, each link of the autonomic system has its own representation in the central nervous system, in particular in the brain stem. A clinical model for understanding the role of the substantia nigra in regulating blood flow is Parkinson's disease, a disease characterized by the progressive loss of dopaminergic neurons in the substantia nigra. Experimental studies modeling this disease indicate a weakening of the sympathetic component of the regulation of blood pressure and heart rate during degeneration of the substantia nigra [39].
Thus, this work shows that damage to the dopaminergic structure of the substantia nigra and the cholinergic structure of the Meynert nucleus area in patients with DAP is associated with a more pronounced and long-lasting impairment of the autoregulation of cerebral blood flow. The data obtained indicate the existence in humans of neurogenic mechanisms regulating cerebral vascular tone that contribute to changes in ICP. Damage to these regulatory links as a result of injury is associated with a more severe and prolonged period of impaired autoregulation of cerebral blood flow, which may require longer monitoring and correction of ICP.
The results of the work show the presence of different patterns of brain damage in patients with impaired and intact mechanisms of cerebral autoregulation with the same severity and clinical form of TBI. Our data, from the clinical side, indirectly confirm the results of previously conducted experimental studies regarding the presence of direct neuronal mechanisms for regulating vascular tone. However, these data should be interpreted with caution; they are preliminary and only lift the curtain on the complex mechanisms of regulation of cerebral blood flow in acute brain pathology. Of course, further, more detailed research in this direction is needed, including to identify all parts of the neuronal regulatory system.
The work was supported by the Russian Foundation for Basic Research grant No. 16−04−01472.
The authors declare no conflict of interest.
Local regulation
If we consider the regulation of blood circulation briefly, it can be noted that this process occurs both at the level of individual organs and in the body as a whole. They have several differences.
Blood brings oxygen to cells and removes waste elements from their vital functions. Local regulation processes are associated with maintaining basal vascular tone. Depending on the intensity of metabolism in a particular system, this indicator may vary.
The walls of blood vessels are covered with smooth muscle. They are never in a relaxed state. This tension is called vascular muscle tone. It is provided by two mechanisms. This is myogenic and neurohumoral regulation of blood circulation. The first of these mechanisms is the main one in maintaining vascular tone. Even if there are absolutely no external influences on the system, the residual tone is still preserved. It was called basal.
This process is ensured by the spontaneous activity of vascular smooth muscle cells. This voltage is transmitted throughout the system. Each cell transmits excitation to the other. This provokes the occurrence of rhythmic fluctuations. When the membrane becomes hyperpolarized, spontaneous excitations disappear. At the same time, muscle contractions disappear.
During metabolism, cells produce substances that have an active effect on vascular smooth muscles. This principle is called feedback. When the tone of the precapillary sphincters increases, blood flow in such vessels decreases. The concentration of metabolic products increases. They help dilate blood vessels and increase blood flow. This process is repeated cyclically. It belongs to the category of local regulation of blood circulation in organs and tissues.
results
MRI analysis data showed that all patients in the analyzed sample had signs of diffuse brain damage involving the hemispheric and in 29 (78.4%) of 37 patients - brainstem structures. At the same time, the small number of patients in the groups with intact and impaired autoregulation did not allow us to identify significant differences in the outcomes according to the GOS severity of injury according to the GCS. The groups did not differ from each other in gender and age characteristics. Localization and depth of brain damage assessed according to the classifications of R. Firsching et al. [22] and N.E. Zakharova et al. [23], also did not differ significantly between the comparison groups (see Table 1).
Prevalence of damage to brain stem and subcortical structures in impaired AMB
Next, a statistical analysis was carried out on the frequency of occurrence of unilateral or bilateral damage to each brain structure in patients with impaired (group 2) and intact (group 1) autoregulation (Table 2).
Table 2. Frequency of unilateral and/or bilateral damage to brain structures in patients with normal and impaired autoregulation Note. *—significant differences between groups.
Statistical analysis of the data showed that in patients with traumatic brain injury, accompanied by disturbances of the AMB in the acute period of injury, in general, damage to the brainstem was observed somewhat more often than in the group of patients with normal autoregulation. Among the studied stem structures, significantly more often ( p
=0.02) in patients of group 2, there was structural damage to the substantia nigra of the midbrain - a structure that is the source of dopaminergic projections for the neostriatum, cingulate cortex, olfactory nuclei, posterior hypothalamus and tonsils of the brain.
The odds ratio was 5.333 (95% CI 1.252, 29.346), sensitivity 62.5% and specificity 76.2%. Also, more frequent damage to the cholinergic structure - the area of Meynert's nucleus - was revealed in patients with impaired autoregulation ( p
= 0.01), and unilateral or bilateral damage to this area of the brain had a fairly high specificity (81%) for patients in this group.
Additionally, the frequency of occurrence of combined damage to the substantia nigra and Meynert's nucleus was assessed, which significantly prevailed in the group with impaired autoregulation ( p
= 0.02). The odds ratio was 7.39 (95% CI 1.043, 65.37), sensitivity 43.8% and specificity 90.5%.
Thus, the analysis showed that impaired autoregulation of cerebral blood flow in patients with brain DAP is often associated with the presence of primary damage to the substantia nigra, the area of Meynert’s nucleus, and their combination (Fig. 2).
Rice. 2. MRI of the brain of patients with severe TBI with damage to the structures of the substantia nigra (a), the area of Meynert’s nucleus (b), combined damage to the substantia nigra and the area of Meynert’s nucleus (c). Damage is indicated by arrows.
Prevalence of damage to subcortical and brain stem structures in long-term autoregulation disorders
To analyze the effect of damage to the subcortical and stem structures of the brain on the parameter of autoregulation of blood flow (PRx), patients were divided into three approximately equal groups depending on the duration of the period of lost autoregulation (PRx>0.2) relative to the entire measurement time: 1) less than 20% time ( n
=17);
2) 20–34% of the time ( n
=10);
3) 35% of the time or more ( n
=10). The results of this analysis are presented in table. 3.
Table 3. Frequency of damage to brain structures in patients with different durations of autoregulation disorders (as a percentage of the duration of ICP measurement) Note.
* — differences between groups 1 and 3; # — differences between the 2nd and 3rd groups. It was found that in patients with lost autoregulation more than 35% of the measurement time, trunk damage was somewhat more likely to be present. In this group of patients, injuries to the substantia nigra were significantly more common ( p
=0.05).
The odds ratio was 5.6 (95% CI 0.785, 45.938), sensitivity and specificity were 70 and 70.6%, respectively. Also in this group, more frequent damage to the Meynert nucleus region was noted ( p
= 0.04). The odds ratio was 7.6 (95% CI 1.006, 68.466), sensitivity and specificity were 70 and 76.5%, respectively.
According to the results of this analysis, significant differences remained in the frequency of damage to the substantia nigra, the area of the Meynert nucleus and their combination between extreme groups (with the duration of impaired autoregulation less than 20% and more than 35% of the measurement time). Thus, the presence of primary damage to the brainstem in the area of the substantia nigra and basal forebrain (Meynert's nucleus area) makes a significant contribution to the disruption of the AUB mechanisms.
Local and central regulation
The mechanisms of regulation of organ blood circulation are subject to two interrelated factors. On the one hand, there is central regulation in the body. However, for a number of organs with a high rate of metabolic processes, this is not enough. Therefore, local regulatory mechanisms are clearly expressed here.
These organs include the kidneys, heart and brain. In those tissues that do not have a high level of metabolism, such processes are less pronounced. Local regulatory mechanisms are necessary to maintain a stable speed and volume of blood flow. The more pronounced the metabolic processes in an organ, the more it needs to maintain a stable inflow and outflow of blood. Even with pressure fluctuations in the systemic bloodstream, its stable level is maintained in these parts of the body.
However, the local regulatory mechanism is still insufficient to ensure rapid changes in blood inflow and outflow. If only these processes existed in the body, they would not be able to ensure correct, timely adaptation to changing external conditions. Therefore, local regulation is necessarily added by processes of central neurohumoral regulation of blood circulation.
Nerve endings are responsible for the processes of innervation of blood vessels and the heart. The receptors that are present in the system respond to different blood parameters. The first category includes nerve endings that respond to changes in pressure in the riverbed. They are called mechanoreceptors. If the chemical composition of the blood changes, other nerve endings react to this. These are chemoreceptors.
Mechanoreceptors respond to stretching of the walls of blood vessels and changes in the speed of fluid movement in them. They are able to distinguish between fluctuations in increasing pressure or pulse jerks.
A single field of nerve endings, which is located in the vascular system, consists of angioreceptors. They accumulate in certain areas. These are reflexogenic zones. They are determined in the sinocarotid, aoral region, as well as in vessels that are concentrated in the pulmonary circulation of blood. When pressure increases, mechanoreceptors create a volley of impulses. They disappear when the pressure decreases. The excitation threshold of mechanoreceptors ranges from 40 to 200 mmHg. Art.
Chemoreceptors respond to an increase or decrease in the concentration of hormones and nutrients inside the vessels. They transmit signals about the collected information to the central nervous system.
Material and methods
The analysis included 37 patients with mTBI (3-8 points on the Glasgow Coma Scale) who were treated in the intensive care unit of the National Medical Research Center for Neurosurgery named after. N.N. Burdenko in the period from 2009 to 2014. The inclusion criteria were met by patients who, according to clinical indications, required monitoring of blood pressure, ICP, CPP and in whom MRI of the brain revealed signs of DAP. Monitoring data was stored, analyzed and calculated using ICM Plus software. In total, the analysis included 23 men and 12 women, whose average age was 28±12.4 years. Seven patients in this group underwent decompressive craniotomy due to diffuse cerebral edema.
In the intensive care unit, patients were given mechanical ventilation, PaCO2 was maintained at 35-45 mm Hg, PaO2 was not lower than 100 mm Hg, sedation and analgesia were administered (propofol 1-3 mg/kg/h or midazolam 10-30 mcg/kg/h, fentanyl 1-2 mcg/kg/h). CPP was maintained above 60 mm Hg. When ICP is above 20 mm Hg. bolus administration of 15% mannitol (0.25-1 g/kg) or Hyperhaes at a dose of 2-3 ml/kg was used.
The Glasgow Coma Scale (GCS) was used to assess the depth of coma [19, 20]. TBI outcomes were assessed using the Glasgow Outcome Scale (GOS) [20, 21]. Brain damage in DAP was assessed using a classification based on MRI data [22]. The location and level of brain damage were assessed using the MRI classification proposed by N.E. Zakharova et al. [23].
All patients underwent ICP monitoring in accordance with international recommendations and the protocol of the Association of Neurosurgeons of the Russian Federation [7, 24]. ICP monitoring was carried out using a Codman ICP Express Monitor and a Codman MicroSensor (Jonson & Johnson Professional, Inc., Raynham, USA). The ICP sensor was implanted into the white matter of the brain through the trefination foramen in the projection of Kocher's point into the premotor zone of the subdominant hemisphere. BUN was assessed by monitoring the cerebral vascular reactivity index PRx [10]. The average duration of PRx monitoring was 7 days. The ratio of the duration of the period of impaired autoregulation to the total duration of monitoring of this parameter was also assessed.
BP monitoring was performed using direct measurement through an arterial catheter placed in the radial, femoral, or dorsalis pedis arteries.
Based on the calculation of the average PRx coefficient for the entire observation period when monitoring ICP and CPP, two groups of patients were identified:
1st group
— with preserved AUB — PRx [–1; 0];
2nd group
— with partially or completely lost AUB — PRx (0; 1].
The characteristics of patients in each group are presented in Table. 1.
Table 1. Comparative analysis of groups with intact and impaired AUB Note. Abbreviations: GCS - Glasgow Coma Scale, GOS - Glasgow Outcome Scale, DAP - diffuse axonal injury, ICP - intracranial pressure, ICH - intracranial hypertension, PRx - cerebral vascular reactivity coefficient. Group 1 consisted of 19 patients. In 16 (84.2%) of them, the cause of injury was a traffic accident. Brain stem damage was diagnosed in 15 (78.9%) patients during MRI.
Group 2 was represented by 18 patients. In 15 of them, the cause of injury was a traffic accident. Seven patients underwent decompressive trepanation due to diffuse cerebral edema. In 15 (83.3%) patients in this group, brain stem damage was visualized during MRI.
Neuroimaging methods.
MRI of the brain was performed on a 3 T GE tomograph in standard modes (T1, T2, FLAIR) and SWI/T2* GRE, DWI modes, which made it possible to evaluate both ischemic and small hemorrhagic focal lesions. In each patient, according to MRI data, damage to individual subcortical structures and areas of the brain stem, which are projections of the main neurotransmitter systems of the brain, was assessed (Fig. 1),
Rice.
1. Location of brain structures included in factor analysis. NC - caudate nucleus, Put - putamen, GPi - internal segment of the globus pallidus, GPe - external segment of the globus pallidus, Tha - thalamus, SN - substantia nigra, VTA - ventral tegmental area, MN - Meynert's nucleus, LDT - laterodorsal tegmental nucleus, PPN - pedunculopontine nucleus, NR - red nucleus, LC - locus coeruleus. Dopaminergic brain structures are highlighted in red, noradrenergic in blue, cholinergic in green, glutamatergic in orange, and GABAergic brain structures in purple. as well as areas of damage to the frontal lobes (mediobasal, pole and dorsolateral sections). Statistical methods.
Data processing was carried out in the Statistica 8.0 software package.
(StatSoft Inc, USA). In all cases, nonparametric tests were used for statistical evaluation. To analyze qualitative characteristics, Fisher's test ( F
) was used; odds ratios, sensitivity and specificity of each factor were calculated to assess the influence of anatomical factors on the risk of developing unstable hemodynamics.
Results were considered statistically significant at p
< 0.05.
Central mechanisms
The circulatory regulation center regulates the amount of ejection from the heart, as well as vascular tone. This process occurs due to the general functioning of the nervous structures. They are also called the vasomotor center. It includes different levels of regulation. Moreover, there is a clear hierarchical subordination here.
The center for regulating blood circulation is located in the hypothalamus. Subordinate structures of the vasomotor system are located in the spinal cord and brain, as well as in the cerebral cortex. There are several levels of regulation. They have blurred boundaries.
The spinal level represents neurons that are located in the lumbar and lateral horns of the thoracic spinal cord. The axons of these nerve cells form fibers that constrict blood vessels. Their impulses are supported by the underlying structures.
The bulbar level is a vasomotor center located in the medulla oblongata. It is located at the bottom of the 4th ventricle. This is the main center for regulating the blood circulation process. It is divided into pressor and depressor parts.
The first of these zones is responsible for increasing pressure in the riverbed. At the same time, the frequency and strength of contractions of the heart muscle increase. This helps to increase the IOC. The depressor zone performs the opposite function. It reduces pressure in the arteries. At the same time, the activity of the heart muscle also decreases. Reflexively, this area inhibits neurons that belong to the pressor zone.
Treatment and prevention of cerebrovascular accidents
Stroke is one of the leading causes of death and the most common cause of disability.
Stroke prevention includes:
- Timely diagnosis and treatment of diabetes.
- Treatment of arterial hypertension: sodium restriction, regular use of medications to lower blood pressure.
- Controlling blood cholesterol levels: proper nutrition, weight loss, use of statins.
- Diagnosis and identification of the causes of transient ischemic attacks (TIA).
- Avoiding stress and psycho-emotional overload.
- Timely diagnosis and treatment of vascular atherosclerosis.
- Maintaining a sleep schedule and physical activity.
- Diet selection.
- Strengthening the walls of blood vessels, both with medications and preventive measures - hardening, gymnastics, taking antioxidants and vitamins.
- Quitting smoking.
Adequate treatment of cerebral circulatory disorders begins with examination and establishment of the exact cause of the pathology. You cannot self-medicate and haphazardly reduce blood pressure - this disrupts the coordinated work of the cardiovascular system, increases the load on the walls of blood vessels, which leads to the formation of aneurysms, stenoses and, as a result, impaired renal filtration, thereby aggravating the course of hypertension, increasing the risk of strokes and heart attacks . When antihypertensive drugs are taken irregularly and incorrectly, cerebral perfusion pressure drops, which causes blockage of blood vessels and brain hypoxia. The presence of the slightest symptoms of MV disturbance is a reason to immediately consult a doctor.
Unfortunately, against the background of constant stress, poor nutrition, untimely examination, uncontrolled use of medications, cerebrovascular accidents began to affect young people. Sometimes, frequent headaches may indicate the onset of changes in the blood vessels of the brain, and therefore taking painkillers can complicate timely diagnosis and lead to complications.
The initial examination of the cerebral blood supply should include:
- Clinical blood test
- General urine analysis
- Biochemical blood test, in particular lipid profile with determination of the atherogenic index, determination of blood glucose
- Coagulogram
- Ultrasound of brachycephalic and transcranial vessels of the neck and brain
- Ultrasound of the heart (ECHO-CG)
- Ultrasound of the kidneys and adrenal glands
- Measuring anthropometric data and calculating body mass index
- Consultation with an ophthalmologist, with mandatory examination of the fundus vessels
- Consultation with a cardiologist
- Neurologist consultation
This is a mandatory list for a basic study to determine the risks or degree of cerebrovascular accident, but the doctor, based on the examination data, anamnesis and research results, can recommend more in-depth examinations, including CT and MRI, to establish the severity of the condition, establish the correct diagnosis and select therapeutic and preventive measures.
For more detailed consultation, examination and examinations, please contact medical specialists.
Other levels of regulation
Neurohumoral regulation of blood circulation is ensured by the work of other levels. They occupy a higher position in the hierarchy system. Thus, the vasomotor center is influenced by the hypothalamic level of regulation. This influence is of a downward nature. The hypothalamus also distinguishes between pressor and depressor zones. This can be considered as a duplicate of the bulbar level.
There is also a cortical level of regulation. There are zones in the cerebral cortex that have a descending effect on the center located in the medulla oblongata. This process is the result of a comparison of data received from higher receptor zones based on information from various receptors. This shapes the implementation of behavioral reactions, the cardiovascular component of emotions.
The listed mechanisms form the central link. However, there is another mechanism of neurohumoral regulation. It is called the efferent link. All parts of this mechanism enter into complex interaction with each other. They consist of different components. Their relationship allows you to regulate blood flow in accordance with the existing needs of the body.
Arterial blood supply
A feature of cerebral circulation is the high intensity and almost constant volume of blood, which within the cranium is about 75 ml. More than 20% of the blood during a heartbeat enters the brain tissue. The anatomy of the arteries running in the brain is represented by the elements:
- Carotid system. The pool is formed by the carotid arteries running in parallel. The carotid artery divides into branches. Carotid blood flow is fast and direct.
- Vertebro-basilar system. The pool is created by the vertebral and basilar arteries. Vertebro-basilar blood flow is slow.
- Circle of Willis. Provides blood saturation of areas of the cerebrum. The circle is formed by the branches of the carotid artery, which feed the frontal, parietal, and temporal lobes.
- Circle of Zakharchenko. It lies within the inner surface of the medulla oblongata. The contour is formed by two pairs of arteries: vertebral and anterior spinal.
The walls of the small arteries running in the head and neck are three-layered. They consist of layers - internal (endothelium), middle (smooth muscle) and external (connective tissue). A complete circle of blood circulation is completed in 4-5 seconds.
Nervous mechanism
Nervous regulation of blood circulation is part of the efferent link of the global system that controls these processes. This process is carried out through three components:
- Sympathetic preganglionic neurons. Located in the lumbar region and anterior horns of the spinal cord. They are also detected in the sympathetic ganglia.
- Parasympathetic preganglionic neurons. These are the nuclei of the vagus nerve. They are located in the medulla oblongata. This also includes the nuclei of the pelvic nerve, which is located in the sacral part of the spinal cord.
- Efferent neurons of the metasympathetic nervous system. They are needed for hollow organs of the visceral type. These neurons are located in ganglia of the intramural type of their walls. This is the final path along which central efferent influences move.
Almost all vessels are subject to innervation. This is not typical only for capillaries. The innervation of the arteries corresponds to the innervation of the veins. In the second case, the neuron density is lower.
The neurohumoral regulation of blood circulation can be clearly traced to the very sphincters of the capillaries. They end on the smooth muscle cells of these vessels. Nervous regulation of capillaries manifests itself in the form of efferent innervation through the free diffusion of metabolites directed to the walls of blood vessels.
Venous system
Veins are distinguished by thin walls of low elasticity and elasticity. The muscle layer is poorly developed. Large veins contain valves that allow blood to flow in one direction—toward the heart. The blood supply to parts of the brain includes venous sinuses - channels running between the lobes of the meninges.
The sinuses are involved in the process of reabsorption (reverse absorption) of cerebrospinal fluid from the subarachnoid space (located between the pia mater and the arachnoid mater). When intracranial pressure increases, the outflow of venous blood occurs through emissary and diploic veins. Venous blood is dark. It contains carbon dioxide and metabolic products, but there is practically no oxygen.
Endocrine regulation
Regulation of the circulatory system can be accomplished through endocrine mechanisms. The main role in this process is played by hormones that are produced in the brain and cortical layers of the adrenal glands, the pituitary gland (posterior lobe), and the juxtaglomerular renal apparatus.
Adrenaline has a vasoconstrictor effect on the arteries of the skin, kidneys, digestive organs, and lungs. At the same time, the same substance can produce the opposite effect. Adrenaline dilates blood vessels that pass through the skeletal muscles and the smooth muscles of the bronchi. This process promotes blood redistribution. With strong excitement, anxiety, and tension, blood flow increases in the skeletal muscles, as well as in the heart and brain.
Norepinephrine also has an effect on blood vessels, allowing blood to be redistributed. When the level of this substance increases, special receptors react to it. They can be of two types. Both varieties are present in vessels. They control the process of narrowing or expanding the duct.
Considering the physiology of the regulation of blood circulation, we should consider other substances that affect the entire process. One of them is aldosterone. It is produced by the adrenal glands. It affects the sensitivity of the walls of blood vessels. This process is controlled by changing the absorption of sodium by the kidneys, salivary glands, and gastrointestinal tract. The vessels become larger or less susceptible to the effects of adrenaline and norepinephrine.
A substance such as vasopressin helps to narrow the walls of the arteries in the lungs and peritoneal organs. At the same time, the vessels of the heart and brain react to this by expanding. Vasopressin also performs the function of redistributing blood in the body.
Functions of arteries
Healthy arteries provide normal blood supply to areas of the brain. Blood saturated with oxygen moves through the arteries. Arterial blood has a bright, scarlet color. The main function of the arterial network is the delivery of oxygen and nutrient substrate to organs, tissues and cells. If for any reason the patency of the arteries is impaired, a state of hypoxia (oxygen starvation) develops, which provokes ischemia, tissue necrosis (death), stroke, and cerebral infarction.
Signs, causes and methods of treatment for blood supply disorders that occur in the tissues of the spinal cord and brain regions of the head are studied by the branch of medicine - neurology. Damage to individual elements of the cerebral blood flow system does not always manifest itself with severe symptoms.
Thanks to high compensatory abilities, natural regulation of blood supply processes occurs. It has been scientifically proven that in order for hemodynamic and neurological disorders to manifest themselves, the vascular lumen of a major artery must narrow by more than 50% against the background of narrowing of many arteries within one basin. The main causes of circulatory dysfunction:
- Occlusion (impaired patency) of elements of the arterial and venous circuits.
- Aneurysm. Pathological expansion of a section of the artery against the background of loss of the ability to restore its previous shape.
- Thrombosis. Partial or complete blockage of the vascular lumen by a blood clot or cholesterol plaque.
Pathological changes and deformations of vascular walls can be congenital or acquired. Abnormal development of the arterial network is common and is not always accompanied by disruptions in the functioning of the circulatory system. Acquired ones arise as a result of the influence of factors:
- infectious diseases – encephalitis, abscess, meningitis;
- traumatic brain injuries;
- damage to the cardiovascular system;
- chronic stress;
- obesity, endocrine system disorders;
- arterial hypertension;
- osteochondrosis, spinal diseases.
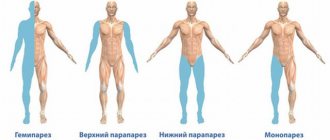
The specificity of the symptoms shows in which part of the brain the disturbances occurred. Pathological changes in the anterior artery are accompanied by motor dysfunction. Signs are a disorder of gross and fine motor skills, abnormal gait, disturbances in facial expressions, gestures, and speech, characteristic of motor aphasia. Impaired blood flow in the middle artery leads to a brachiofacial type of paresis (partial paralysis) and hemihypesthesia - a decrease in the sensitivity of certain parts of the body.
Damage to the tissues of the trunk of the carotid artery is manifested by hemianopsia (bilateral blindness in a separate area of the visual field), disorders of the psycho-emotional sphere, deterioration of the ability to concentrate, impaired memory and fine motor skills. Total pathologies threaten deformation of the walls and structures of the basilar artery. They are expressed by Filimonov's syndrome - tetraplegia (partial or complete paralysis of the limbs) while maintaining consciousness and eye movement function.
Other components of endocrine regulation
Regulation of endocrine-type blood circulation is possible with the participation of other mechanisms. One of them provides a substance called angiotensin-II. It is formed during the breakdown of angiotensin-I enzymes. This process is influenced by renin. This substance has a strong vasoconstrictor effect. Moreover, it is much more powerful than the effects of the release of norepinephrine into the blood. However, unlike this substance, angiotensin-II does not provoke the release of blood from the depot.
This effect is ensured due to the presence of receptors sensitive to the substance only in the arterioles at the entrance to the capillaries. They are located unevenly in the circulatory system. This explains the heterogeneity of the effects of the substance presented in different parts of the body. Thus, a decrease in blood flow with an increase in the concentration of angiotensin-II is determined in the skin, intestines, and kidneys. At the same time, blood vessels dilate in the brain, heart, and adrenal glands. In the muscles, the change in blood flow in this case will be insignificant. If the doses of angiotensin are very large, blood vessels in the brain and heart may narrow. This substance, in combination with renin, forms a separate regulatory system.
Angiotensin can also have indirect effects on the endocrine system, as well as the autonomic nervous system. This substance stimulates the production of adrenaline, norepinephrine, and aldosterone. This enhances the vasoconstrictor effects.
Local hormones (serotonin, histamine, bradykinin, etc.), as well as biologically active compounds, can also dilate blood vessels.
Regulation of regional blood circulation.The adaptation of local blood flow to the functional needs of organs is carried out mainly by changing the resistance to blood flow, i.e. by regulating hydrodynamic resistance. Since resistance is inversely proportional to the radius of the vessels to the fourth (4) power, changes in their lumen have a much greater effect on the amount of blood flow in the organ than changes in perfusion pressure. The lumen of blood vessels is regulated by local mechanisms, as well as nervous and humoral factors.
Local regulatory mechanisms
.
The degree of contraction of vascular smooth muscle is directly influenced by certain substances necessary for cellular metabolism (for example, oxygen) or produced during metabolism. Taken together, they all constitute metabolic autoregulation of peripheral circulation, the most important significance of which is that it adapts local blood flow to the functional activity of the organ. In this case, vasodilatory effects predominate, dominating the nervous vasoconstrictor effects.
Lack of oxygen
Vasodilation also occurs with local CO tension and the concentration of hydrogen ions, as well as the accumulation of lactic acid, ATP, AMP, ADP and adenosine, an increase in the concentration of potassium ions, etc.
Myogenic autoregulation
Some vessels are capable of maintaining a constant volumetric blood flow rate in the organ despite significant pressure fluctuations. This ability can be considered one of the types of myogenic (mechanogenic) autoregulation: it is caused by the reaction of smooth muscles to mechanical stress (the Baylis effect): when stretched, smooth muscles contract, and even to a greater extent than is necessary to maintain the same length. The higher the pressure inside the vessel, the stronger the smooth muscles contract; As a result, with increasing pressure, the blood flow speed either does not change or increases slightly. This mechanism stabilizes the blood supply to the organ. In some organs, the volumetric velocity of blood flow does not change when pressure fluctuates from 120 to 200 mm Hg. Art. A classic example of such vessels are the renal vessels. Myogenic self-regulation is also characteristic of the vessels of the brain, myocardium, liver, intestines and skeletal muscles. It was not found in skin vessels. The myogenic response does not depend on autonomic influences and therefore it persists even after transection of the vasomotor nerves. For more information on the mechanisms of nervous and humoral regulation of vascular tone, see the textbook material.
Peculiarities of blood supply to individual organs in humans
.
Coronary blood supply.
At rest, the value of coronary blood flow in humans is approximately 0.8-0.9 ml per 1 g of myocardial tissue per minute, which for a heart weighing 300 g is about 250 ml/min, which is approximately 4-5 percent of the minute blood volume. With intense muscular work, blood flow can increase 4 times. Coronary blood flow, unlike blood circulation in other organs, undergoes significant fluctuations corresponding to periods of heart activity. These fluctuations are caused by both the pulsating nature of the pressure in the aorta (from which the coronary arteries arise) and changes in tension in the heart wall. Under the influence of this tension, the vessels of the inner and middle walls of the myocardium are compressed, as a result of which during systole the blood flow in the left coronary artery completely stops, while in the right the blood flow changes depending on the pressure in the aorta. In diastole, the volumetric velocity of blood flow in the basin of the left and right coronary arteries is maximum. Thus, the blood flow, and consequently the supply of oxygen to the myocardium, undergoes periodic fluctuations. It is minimal in systole and maximal in diastole. At the same time, the energy needs of myocardial cells change in the opposite way: they increase during the contraction phase and decrease during the relaxation period. There are 2 mechanisms that completely satisfy the energy needs of the myocardium under normal conditions, despite the decrease in oxygen delivery during systole. One of them is that myoglobin plays the role of a short-term oxygen supply. The oxygen stored in this depot supports tissue respiration of cells during systole. The second mechanism boils down to the fact that the increase in myocardial energy demand at the time of heart contraction is satisfied due to its reserves (ATP and creatine phosphate). During diastole, due to a significant increase in blood flow, myoglobin is again completely saturated with oxygen, and cellular energy reserves are replenished; at the same time, during this period the use of oxygen and energy substrates by the heart is very insignificant. During physical activity, additional difficulties are created for the normal supply of oxygen to the myocardium. The heart under these conditions needs greater oxygen supply. At the same time, as a result of an increase in heart rate, the duration of diastole decreases significantly. In this regard, exercise tolerance is limited to a maximum heart rate of approximately 200 beats per minute. The ECG under these conditions often shows typical changes characteristic of myocardial hypoxia.
Regulation of coronary blood flow.
Even at rest, the heart extracts more oxygen from the blood than other organs. The extraction of oxygen by the heart is 0.14 ml/l from arterial blood containing 0.2 ml of oxygen in 1 ml (i.e., the oxygen utilization rate in the heart is about 70%, while other organs at rest are 30-40%) . In this regard, the increase in the heart's oxygen demand during exercise cannot be achieved by increasing its extraction. The increased myocardial oxygen demand is satisfied by increasing coronary blood flow. This increase is due to the expansion of the coronary vessels, i.e. reducing their hydrodynamic resistance. It is generally accepted that the most powerful stimulus for dilatation of coronary vessels is a lack of oxygen: dilatation of coronary vessels occurs already with a decrease in oxygen content by 0.01 ml in 1 ml of blood. The effect of hypoxia on coronary blood flow was confirmed in a breath-hold test: in this case, a significant increase in blood supply to the heart muscle occurs. The direct influence of autonomic nerves on the coronary vessels is difficult to assess, because these nerves simultaneously influence other parameters of the heart. Various researchers express opposing points of view on this issue. This issue is discussed in more detail in specialized literature.
Pulmonary circulation
The lungs are supplied with blood from both circulation circles: the small circle delivers venous blood through the pulmonary artery to the capillaries of the pulmonary alveoli for gas exchange, and the large circle delivers arterial blood through the bronchial arteries to nourish the lung tissue. In various parts of the vascular bed of the lungs, arteries and veins are much shorter, and their diameter, as a rule, is significantly larger compared to the vessels of the systemic circulation. The walls of the large arteries of the lungs are relatively thin, while the small arteries have thick walls with a developed muscle layer. The diameter of the pulmonary capillaries is about 8 microns, the diameter of the arterioles can reach 80 microns (for comparison: the diameter of the capillaries and arterioles of the systemic circulation is 3-7 and 15-60 microns, respectively). In this regard, the resistance to blood flow created by the vessels of the pulmonary circulation is approximately 10 times less than in the systemic circulation. This allows the right ventricle to work with less power. In a healthy person, the pressure in the pulmonary vessels is relatively low. Systolic pressure in the pulmonary artery is 25-30 mm Hg. Art., diastolic - 5-10 mm Hg. Art., pulse - 15-20, average - 13 mm Hg. The pressure in the pulmonary capillaries is 6.5 mm Hg, in the left atrium - 55 mm Hg. Due to the greater distensibility of the pulmonary vessels, the volume of circulating blood in them can change in the direction of decrease or increase, and these fluctuations can reach 200 ml (with an average content of about 440 ml of blood in the pulmonary circulation). The volume of blood in the pulmonary circulation, together with the end-diastolic volume of the left ventricle, constitutes the so-called central blood volume (about 600-650 ml). This central blood volume represents a rapidly mobilized blood depot. So, if it is necessary to increase the output of the left ventricle within a short period of time, then about 300 ml of blood can come from this depot. As a result, the balance between the output of the right and left ventricles will be maintained until another mechanism is activated - an increase in venous return.
Regulation of pulmonary circulation.
The pulmonary vessels are innervated by sympathetic vasoconstrictor fibers. The vessels of the lungs, like the vessels of the systemic circulation, are under the constant tonic influence of the sympathetic nervous system. When the baroreceptors of the carotid sinus are excited due to an increase in blood pressure, the resistance of the vessels in the pulmonary circulation reflexively decreases, which leads to an increase in blood supply to the lungs and normalization of pressure in the systemic circulation. When the baroreceptors of the pulmonary arteries, located at the base of these arteries in the area of the bifurcation of the pulmonary trunk, are excited, which occurs when the pressure in the pulmonary circulation increases, the pressure in the systemic circulation reflexively decreases due to the slowdown of the heart and the dilation of the systemic vessels (Parin reflex). The physiological significance of this reflex is that, by unloading the pulmonary circulation, it prevents the lungs from overfilling with blood and the development of their edema. When the pressure in the pulmonary artery decreases, on the contrary, the systemic pressure increases, and thus the blood supply to the lungs is normalized.
Local regulation of pulmonary blood flow
.
When the partial pressure of oxygen decreases or the partial pressure of carbon dioxide increases, local constriction of the pulmonary vessels occurs (Eimr-Liljestrand reflex). Thanks to this mechanism, blood flow in individual areas of the lungs is regulated in accordance with the ventilation of these areas, which makes it possible to exclude unventilated alveoli from the blood supply. It must be emphasized that in the event of cessation of ventilation of a significant area of the lung tissue (pneumonia), a reflex spasm of the vessels supplying the affected area occurs. This can lead to a sharp increase in hydrodynamic resistance in the pulmonary circulation, and, as a consequence, to the development of right ventricular failure, especially in young children.
Cerebral circulation.
The average cerebral blood flow rate is approximately 750 ml/min. those. 13% of total cardiac output. The blood supply to gray matter is approximately 4 times greater than white matter and amounts to 0.68-1.1 ml per 1 g of tissue per minute. Blood flow may increase in certain areas of the brain when their activity increases, but overall the blood supply to the brain changes slightly.
Regulation of cerebral blood flow.
The size of the vascular lumen depends mainly on metabolic factors, in particular, on the CO tension in the capillaries and tissues, the concentration of hydrogen ions in the perivascular space and the oxygen tension in the blood. An increase in CO tension is accompanied by a pronounced dilation of blood vessels: for example, when the carbon dioxide tension doubles, cerebral blood flow also approximately doubles. The action of CO is mediated by hydrogen ions released during the dissociation of carbonic acid. Other substances, the accumulation of which increases the concentration of hydrogen ions (lactic acid and other metabolic products), also increase cerebral blood flow. Neurological manifestations of hyperventilation syndrome (dizziness, confusion, convulsions, etc.) are caused, on the contrary, by a decrease in cerebral blood flow as a result of hypocapnia. When oxygen tension decreases, the vessels also dilate, and when oxygen tension increases, they narrow, although in general changes in oxygen tension in the blood have less of an effect on blood flow than shifts in carbon dioxide tension. Myogenic autoregulation is well expressed in the vessels of the brain, therefore, with changes in hydrostatic pressure due to changes in head position, cerebral blood flow remains constant. Thus, the blood supply to the brain is regulated primarily by local metabolic and myogenic mechanisms. The influence of autonomic nerves on cerebral vessels is of secondary importance.
Renal circulation.
The average volumetric velocity of renal blood flow at rest is about 4.0 ml per 1 g of tissue per minute, i.e. in general, for kidneys weighing 300 g, approximately 1200 ml/min, which is about 20% of cardiac output. The peculiarity of the blood supply to the kidneys is the presence of two consecutive capillary networks. The afferent (afferent) arterioles break up into glomerular capillaries, separated from the tubular capillary bed by efferent (efferent) arterioles. Efferent arterioles are characterized by high hydrodynamic resistance. The pressure in the glomerular capillaries is quite high (about 60-70 ml Hg), and in the peritubular capillaries it is relatively low (about 13 mm Hg).
Regulation of renal circulation.
For the vessels of the kidneys, myogenic autoregulatory mechanisms are well developed, thanks to which blood flow and capillary pressure in the nephron area are maintained at a constant level when blood pressure fluctuates from 80-120 to 180-200 mm Hg. The renal vessels are innervated by somatic vasoconstrictor nerves. The tone of these nerves at rest is low. When a person moves to a vertical position, the renal vessels participate in the general vasoconstrictor reaction, which ensures the maintenance of blood supply to the brain and heart. Renal blood flow also decreases during exercise and in high ambient temperatures. This provides compensation for the decrease in blood pressure associated with the expansion of muscle and skin vessels. A characteristic feature of the renal vessels is their low ability to expand, which makes it difficult to increase renal blood flow by reducing hydrodynamic resistance. Therefore, in the event of a decrease in blood supply to the kidneys, mechanisms are launched aimed at increasing perfusion pressure, in particular, the production of renin increases. Activation of the renin-angiotensin system, leading to an increase in systemic pressure, to some extent increases renal blood flow.
Blood supply in skeletal muscles.
The blood flow in skeletal muscle at rest is about 0.03-0.04 ml per 1 g of tissue per minute. Since the total muscle mass is approximately 30 kg, overall muscle blood flow is approximately 900-1200 ml/min, i.e. 15-20% of total cardiac output. At maximum physical activity, muscle blood flow can reach 20-22 l/min with a cardiac output of 25 l, i.e. 80-90% of total blood flow. For trained athletes, this value may be even greater.
Regulation of muscle blood flow
.
The vessels of skeletal muscles are innervated by sympathetic vasoconstrictor and vasodilator fibers. At the end of sympathetic vasoconstrictors, norepinephrine is released, at the end of vasodilators - acetylcholine, therefore, sympathetic vasodilator fibers in skeletal muscles are classified as cholinergic fibers. The role of vasodilator nerves can be illustrated by the fact that in a person preparing for muscular activity, increased sympathetic tone can result in a fourfold increase in muscle blood flow. During muscular work, local metabolic regulatory influences on blood vessels significantly prevail over nervous ones. At the same time, the amount of blood flow is also affected by mechanical compression of the vessels by the contacting muscles. When a muscle contracts, blood flow first decreases, then increases even compared to the initial state. In the relaxation phase, it increases even more; this is the so-called reactive hyperemia, caused by the vasodilating effect of metabolic products. Rhythmic muscle contractions are accompanied by fluctuations in blood flow - a decrease in blood flow during contraction and an increase in the relaxation phase. In this case, the average blood flow speed is always greater than at rest. Thus, with dynamic muscle work, when contractions and relaxations constantly alternate, the muscles fatigue less than with a static load.
Skin circulation.
Even under conditions of neutral ambient temperature (about 20 C for a lightly dressed person), blood flow in different areas of the skin at rest fluctuates significantly. Skin blood flow varies from 0.03 to 0.0 n ml per 1 kg of tissue per minute, or in general, taking into account the weight of the skin 5 kg - from 160 to 500 ml/min or 3-10% of the cardiac output.
Regulation of skin blood flow.
Two different mechanisms are involved in the regulation of cutaneous blood flow, the role of which is different in different areas of the skin. The skin vessels of the acral areas (hands, feet, earlobes) are richly innervated by sympathetic adrenergic vasoconstrictor fibers, which have a relatively high tone at rest and at a neutral temperature. Expansion of these vessels is associated with central inhibition of the tone of the vasoconstrictor nerves. The dilation of the skin vessels of the proximal parts of the limbs and torso occurs predominantly indirectly: it is mediated by the release of bradykinin upon stimulation of cholinergic sweating fibers. The narrowing of all skin vessels is caused by an increase in the tone of sympathetic adrenergic fibers. Due to the high power of the subpapillary venous plexus (about 1500 ml), changes in the tone of the skin veins can be accompanied by significant changes in the blood volume in the skin vessels. Thus, an important function of the skin vessels is to deposit blood.
Skin blood flow and thermoregulation.
The most important function of skin blood flow is participation in the mechanisms of thermoregulation. Under heat stress, the value of total blood flow in the skin can increase up to 3 l/min. However, these changes vary significantly in different areas of the skin. The greatest fluctuations in blood flow are observed in the skin of the distal extremities. So, if Place a finger from cold water into hot water, then the blood flow in it can increase from 0.01 to 1 ml/min per 1 g of tissue, i.e. 100 times or more. The reaction of the skin vessels of the proximal parts of the extremities of the body to a similar effect is much weaker. The increase in skin blood flow under conditions of high external temperature is associated with the opening of many arteriovenous anastomoses, through which part of the blood flows into the veins, bypassing the capillaries. Due to the high thermal conductivity of the skin, this mechanism serves as an extremely effective way of transferring heat through the skin.
Age reactions
There are age-related features of blood circulation regulation. In childhood and adulthood they differ significantly. This process is also influenced by a person’s training. In newborns, sympathetic and parasympathetic nerve endings are clearly expressed. Up to three years of age, the tonic influence of nerves on the heart predominates in children. The center of the vagus nerve is characterized by low tone at this age. It begins to affect blood circulation as early as 3-4 months. However, this process manifests itself more clearly in adulthood. This becomes noticeable at school age. During this period, the child's heart rate decreases.
Having examined the features of blood circulation regulation, we can conclude that this process is complex. Many factors and mechanisms influence it. This allows you to clearly respond to any environmental changes and regulate the supply of vital substances to organs that are currently more loaded.