Structure and functions
The physiology of the nervous system is a complex composite structure.
The neuron is considered the basic structural and functional unit of the nervous system. Its processes form fibers that are excited when exposed and transmit impulses. The impulses reach the centers where they are analyzed. Having analyzed the received signal, the brain transmits the necessary reaction to the stimulus to the appropriate organs or parts of the body. The human nervous system is briefly described by the following functions:
- providing reflexes;
- regulation of internal organs;
- ensuring the interaction of the body with the external environment, by adapting the body to changing external conditions and stimuli;
- interaction of all organs.
The importance of the nervous system lies in ensuring the vital functions of all parts of the body, as well as the interaction of a person with the outside world. The structure and functions of the nervous system are studied by neurology.
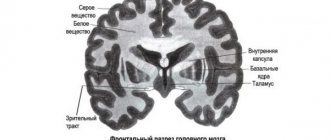
brain matter
- Gray matter is formed by the bodies of neurons and short processes - dendrites
- White matter is formed by long processes of neurons - axons.
- Nervous regulation is carried out by a nerve impulse.
- A nerve impulse is an electrical wave traveling along a nerve fiber.
- Converts irritation into a nerve impulse receptor.
- The activity of the nervous system is reflexive in nature.
- A reflex is the body’s response to stimulation, occurring with the participation of the nervous system.
- A nerve impulse generated by stimulation travels a certain path called a reflex arc.
- Reflex arc - the path along which nerve impulses (nervous excitation) pass from receptors to the executive (working) organ during the implementation of a reflex; includes five departments:
Structure of the central nervous system
The anatomy of the central nervous system (CNS) is a collection of neuronal cells and neural processes of the spinal cord and brain. A neuron is a unit of the nervous system.
The function of the central nervous system is to ensure reflex activity and process impulses coming from the PNS.
The anatomy of the central nervous system, the main unit of which is the brain, is a complex structure of branched fibers.
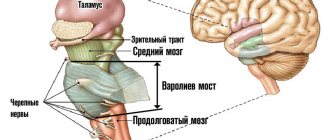
The higher nerve centers are concentrated in the cerebral hemispheres. This is a person’s consciousness, his personality, his intellectual abilities and speech. The main function of the cerebellum is to ensure coordination of movements. The brain stem is inextricably linked with the hemispheres and cerebellum. This section contains the main nodes of the motor and sensory pathways, which ensures such vital functions of the body as regulating blood circulation and ensuring respiration. The spinal cord is the distribution structure of the central nervous system; it provides branching of the fibers that form the PNS.
The spinal ganglion is the site of concentration of sensory cells. With the help of the spinal ganglion, the activity of the autonomic department of the peripheral nervous system is carried out. Ganglia or nerve ganglia in the human nervous system are classified as the PNS; they perform the function of analyzers. Ganglia do not belong to the human central nervous system.
Features of the structure of the PNS
Thanks to the PNS, the activity of the entire human body is regulated. The PNS consists of cranial and spinal neurons and fibers that form ganglia.
The human peripheral nervous system has a very complex structure and functions, so any slightest damage, for example, damage to blood vessels in the legs, can cause serious disruptions to its functioning. Thanks to the PNS, all parts of the body are controlled and the vital functions of all organs are ensured. The importance of this nervous system for the body cannot be overestimated.
The PNS is divided into two divisions - the somatic and autonomic PNS systems.
The somatic nervous system performs double duty - collecting information from the sensory organs, and further transmitting this data to the central nervous system, as well as ensuring the motor activity of the body by transmitting impulses from the central nervous system to the muscles. Thus, it is the somatic nervous system that is the instrument of human interaction with the outside world, as it processes signals received from the organs of vision, hearing and taste buds.
The autonomic nervous system ensures the functions of all organs. It controls the heartbeat, blood supply, and breathing. It contains only motor nerves that regulate muscle contraction.
To ensure the heartbeat and blood supply, the efforts of the person himself are not required - this is controlled by the autonomic part of the PNS. The principles of the structure and function of the PNS are studied in neurology.
Structure of the nervous system
Excitability, irritability and conductivity are characterized as functions of time, that is, it is a process that occurs from irritation to the appearance of an organ response. The propagation of a nerve impulse in a nerve fiber occurs due to the transition of local foci of excitation to adjacent inactive areas of the nerve fiber. The human nervous system has the property of transforming and generating energies from the external and internal environment and converting them into a nervous process.
Structure of the human nervous system: 1-brachial plexus; 2- musculocutaneous nerve; 3rd radial nerve; 4- median nerve; 5- iliohypogastric nerve; 6-femoral-genital nerve; 7- locking nerve; 8-ulnar nerve; 9 - common peroneal nerve; 10- deep peroneal nerve; 11- superficial nerve; 12- brain; 13- cerebellum; 14- spinal cord; 15- intercostal nerves; 16- hypochondrium nerve; 17 - lumbar plexus; 18-sacral plexus; 19-femoral nerve; 20- genital nerve; 21-sciatic nerve; 22- muscular branches of the femoral nerves; 23- saphenous nerve; 24 tibial nerve
The nervous system functions as a whole with the senses and is controlled by the brain. The largest part of the latter is called the cerebral hemispheres (in the occipital region of the skull there are two smaller hemispheres of the cerebellum). The brain connects to the spinal cord. The right and left cerebral hemispheres are connected to each other by a compact bundle of nerve fibers called the corpus callosum.
The spinal cord , the main nerve trunk of the body, passes through a canal formed by the foramina of the vertebrae and stretches from the brain to the sacral spine. On each side of the spinal cord, nerves extend symmetrically to different parts of the body. The sense of touch is, in general terms, provided by certain nerve fibers, countless endings of which are located in the skin.
Departments of the PNS
The PNS also consists of the afferent nervous system and the efferent nervous system.
The afferent region is a collection of sensory fibers that process information from receptors and transmit it to the brain. The work of this department begins when the receptor is irritated due to any impact.
The efferent system differs in that it processes impulses transmitted from the brain to effectors, that is, muscles and glands.
One of the important parts of the autonomic division of the PNS is the enteric nervous system. The enteric nervous system is formed from fibers located in the gastrointestinal tract and urinary tract. The enteric nervous system controls the motility of the small and large intestines. This section also regulates the secretions released in the gastrointestinal tract and provides local blood supply.
Vegetatics
Classifying the nervous systems of people according to their structure is not the only way to divide its departments. The autonomic nervous system, which directly controls the organs of all systems, is of enormous importance. A person cannot consciously control the activity of the vegetative system, but information about how it works sometimes helps to correct well-being in case of vegetative disorders, for example, with a common disease - VSD (vegetative-vascular dystonia).
The activity of the autonomic nervous system is carried out through two antagonistic divisions: sympathetic and parasympathetic. That is, when the sympathetic department is activated, the activity of the parasympathetic automatically stops.
Intrauterine development of the central nervous system
The importance of the nervous system is to ensure the functioning of internal organs, intellectual function, motor skills, sensitivity and reflex activity. The child’s central nervous system develops not only during the prenatal period, but also during the first year of life. Ontogenesis of the nervous system begins from the first week after conception.
The basis for brain development is formed already in the third week after conception. The main functional nodes are identified by the third month of pregnancy. By this time, the hemispheres, trunk and spinal cord have already been formed. By the sixth month, the higher parts of the brain are already better developed than the spinal part.
By the time a baby is born, the brain is the most developed. The size of the brain in a newborn is approximately an eighth of the child’s weight and ranges from 400 g.
The activity of the central nervous system and PNS is greatly reduced in the first few days after birth. This may include an abundance of new irritating factors for the baby. This is how the plasticity of the nervous system manifests itself, that is, the ability of this structure to be rebuilt. As a rule, the increase in excitability occurs gradually, starting from the first seven days of life. The plasticity of the nervous system deteriorates with age.
Types of CNS
In the centers located in the cerebral cortex, two processes simultaneously interact - inhibition and excitation. The rate at which these states change determines the types of nervous system. While one part of the central nervous system is excited, another is slowed down. This determines the features of intellectual activity, such as attention, memory, concentration.
Types of the nervous system describe the differences between the speed of inhibition and excitation of the central nervous system in different people.
People may differ in character and temperament, depending on the characteristics of the processes in the central nervous system. Its features include the speed of switching neurons from the process of inhibition to the process of excitation, and vice versa.
The types of nervous system are divided into four types.
- The weak type, or melancholic, is considered the most predisposed to the occurrence of neurological and psycho-emotional disorders. It is characterized by slow processes of excitation and inhibition. The strong and unbalanced type is choleric. This type is distinguished by the predominance of excitation processes over inhibition processes.
- Strong and agile - this is a type of sanguine person. All processes occurring in the cerebral cortex are strong and active. A strong but inert, or phlegmatic type, is characterized by a low speed of switching nervous processes.
The types of the nervous system are interconnected with temperaments, but these concepts should be distinguished, because temperament characterizes a set of psycho-emotional qualities, and the type of the central nervous system describes the physiological characteristics of the processes occurring in the central nervous system.
CNS protection
The anatomy of the nervous system is very complex. The central nervous system and PNS suffer due to the effects of stress, overexertion and lack of nutrition. For the normal functioning of the central nervous system, vitamins, amino acids and minerals are necessary. Amino acids take part in brain function and are building materials for neurons. Having figured out why vitamins and amino acids are needed and why, it becomes clear how important it is to provide the body with the necessary amount of these substances. Glutamic acid, glycine and tyrosine are especially important for humans. The regimen for taking vitamin-mineral complexes for the prevention of diseases of the central nervous system and PNS is selected individually by the attending physician.
Damage to bundles of nerve fibers, congenital pathologies and abnormalities of brain development, as well as the action of infections and viruses - all this leads to disruption of the central nervous system and PNS and the development of various pathological conditions. Such pathologies can cause a number of very dangerous diseases - immobility, paresis, muscle atrophy, encephalitis and much more.
Malignant neoplasms in the brain or spinal cord lead to a number of neurological disorders. If an oncological disease of the central nervous system is suspected, an analysis is prescribed - histology of the affected parts, that is, an examination of the composition of the tissue. A neuron, as part of a cell, can also mutate. Such mutations can be identified by histology. Histological analysis is carried out according to the doctor’s indications and consists of collecting the affected tissue and its further study. For benign formations, histology is also performed.
The human body contains many nerve endings, damage to which can cause a number of problems. Damage often leads to impaired mobility of a body part. For example, an injury to the hand can lead to pain in the fingers and impaired movement. Osteochondrosis of the spine can cause pain in the foot due to the fact that an irritated or compressed nerve sends pain impulses to receptors. If the foot hurts, people often look for the cause in a long walk or injury, but the pain syndrome can be triggered by damage to the spine.
If you suspect damage to the PNS, as well as any related problems, you should be examined by a specialist.
Be smart!
Action potential (AP) is the electrical component of excitation of nerve cells and most muscle cells (fibers). PD occurs in response to stimulation of sufficient strength. PD is a very fast, short-term electrical process. Therefore, to record it, a cathode oscilloscope with a wideband amplifier is required.A classic study of the parameters and mechanism of AP was carried out in the works of Hodgkin and Huxley on the squid giant axon with intracellular potential removal and intracellular stimulation. Two very thin coaxial wire electrodes were inserted into this nerve fiber (fiber diameter d 0.5-1 mm), one of which recorded the electrical potential relative to the external electrode, the other was irritating: through it current impulses of one direction or another were supplied to the fiber (Fig. .3.3 ). When a short and weak impulse of the outgoing current was applied (its direction is determined for positive charges), the intracellular electrode recorded a short-term drop in the membrane potential (MP), in shape and strength corresponding to the current impulse, but with smoothed leading and trailing edges (the smoothness of the fronts is determined by the membrane capacitance) . This is the so-called electrotonic potential (EP). When a slightly stronger impulse of the outgoing current is applied, an additional depolarization is added to the electrotonic potential, called the subthreshold or “local” response (LR). It is called local because it does not spread either in these or in natural conditions. And if the stimulus is further intensified and a critical level of depolarization (CLD) is reached, then an action potential arises (Fig.). When pushing an incoming current of any strength, only an electrotonic potential is obtained.
In an action potential (AP), a peak (spike) and trace potentials are distinguished.
Fig.3.3. Schemes of techniques used on various nerve fibers to study their electrogenesis
a - intracellular stimulation and discharge of potentials of the squid giant axon during coaxial introduction of electrodes.
b - irritation and diversion of potentials from a single node of Ranvier, isolated by two air gaps (“bridges”).
The AP peak represents a transient inversion of the intracellular potential. It has a very fast S-shaped rising phase and a slightly slower falling phase. The total duration of the peak in this object is approximately 3 ms. The peak amplitude is ∼110 mV, i.e., it exceeds the MPP (-70 mV) by 40 mV. This difference is called overshoot (“overshoot”). Following the AP peak, significantly weaker and longer-lasting negative and then positive trace potentials are recorded (Fig. 3.4). AP has standard parameters that do not depend on the strength of the stimulus that caused the AP.
Fig.3.4. Scheme for recording the main electrophysiological phenomena in a nerve fiber.
MP - resting membrane potential, PD - action potential, LO - local response, CUD - critical level of depolarization, SN - trace negativity, SP - trace positivity, AET - anelectrotonic potential, KET - catelectrotonic potential.
When a giant nerve fiber is stimulated by an outgoing current through a coaxial electrode, all points of this nerve conductor are stimulated almost uniformly, and AP simultaneously arises and develops in them. Such PD, in essence, does not extend and is called “membrane”. Under natural conditions, PD only occurs locally and then spreads (is carried out) along the fiber. This is a spreading PD. Membrane PD is somewhat simpler than propagating one, which makes it more convenient for analysis. Note that in myelinated fibers, membrane AP is obtained when working at an isolated single node of Ranvier.
What is the nature of an action potential?
First of all, AP is an electrical phenomenon that occurs at the plasma membrane and in connection with its activity. Baker, Shaw, and Hodgkin showed that an almost normal AP also occurs in a perfused giant axon, devoid of axoplasm, upon electrical stimulation of its membrane.
The reason for the development of PD is the opening of voltage-dependent channels in the membrane caused by electrical stimulation, or more precisely by critical depolarization of the membrane.
Already in 1939, Kohl and Curtis found that with the development of AP, the transverse impedance of the squid axon sharply decreases due to a drop in its RM (from 1000 to 25 Ohm • cm2) with a constant Cm. As subsequent studies showed, this is associated with the discovery of voltage-dependent sodium and potassium channels. Note that the leakage channels involved in the formation of the MPP are potential independent. The opening of voltage-gated channels leads to the passive movement of the corresponding ions along their electrochemical gradients. Moreover, the entry of sodium ions into the fiber ensures the ascending phase of the AP peak, depolarization and inversion of the potential on the membrane, and the somewhat delayed exit of potassium ions is involved in the creation of the descending phase of the peak, repolarization. With the development of the AP peak, the ratio Pk: PNA: PCI becomes equal to 1: 20: 0.45 (at rest it = 1: 0.04: 0.45). The connection between the development of the AP peak and the Na+ current is proven by the following circumstances: 1) the direct dependence of the AP amplitude on the electrochemical gradient of Na+ on the membrane, 2) the reliable transition of labeled 24Na from the medium into the fiber upon its excitation, and in an amount proportional to the number of APs.
The connection between the descending phase of AP and the K+ current is proven by the dependence of the course of this phase on the electrochemical gradient of K+ on the membrane.
A detailed analysis of changes in membrane permeability for Na+ and K+ ions, based on measurements of the currents of these ions, became possible thanks to the use of the method of fixing (“clamp”) the electrical potential of the membrane, first used by Col and Marmont (1949). Note that membrane currents (at given concentration gradients) depend on both ionic permeability and membrane potential. Therefore, membrane currents can accurately characterize changes in P only at MP = const.
The method for fixing the membrane potential is as follows. The membrane of a giant fiber (or any other cell) is connected to an electronic circuit using intracellular and external electrodes. The main part of this circuit is a differential amplifier that compares the MF with the potential E set from an external source. The current (I) at the output of the amplifier is determined in direction and magnitude by the E-MF difference supplied to the input. This current (/) flows through the membrane and creates an additional potential jump on it, reducing the absolute value of the E-MF difference. With a large gain of the differential amplifier and a small value of R in the circuit supplying the current, the compensating potential jump on the membrane can be very close in magnitude to the E-MF difference and thus, as a result, the MP can become approximately equal to E.
In this situation, with sufficient speed of the system (at τ<20 μs), any, even weak, random displacement of the MP is immediately compensated. Thus, the MP is fixed at the value E and at the same time controlled by changing this value E.
The method of fixing the potential on the membrane excludes any displacements of the MP, including action potentials. But it allows you to record and study transmembrane currents that arise when artificially changing the MP (E). If the fixed MF is equal to the resting potential, then there is practically no transmembrane current. If the MF is increased abruptly, then only a very short capacitive current directed inward appears, followed by a constant leakage current of polyionic, mainly potassium, nature. But if the MF is abruptly reduced, then following the discharge current of the membrane capacitance, against the background of a leakage current directed outward, an additional short (so-called “fast”) current appears inward and is followed by a longer (so-called “slow” current) outward. The last two currents are ionic currents flowing through sodium and potassium channels opened by depolarization. If the sodium gradient on the membrane is eliminated by replacing part of Na+ with choline, or the MP is increased to a value equal to ENа, then the picture is transformed: the fast, inwardly directed current disappears, but a slow outwardly directed current is revealed in an uncomplicated form. The same can be achieved by specific blockade of sodium channels using tetrodotoxin (TTX). Thus, the fast inward current is the sodium current. A picture of its development can be obtained by geometrically subtracting the slow ion current from the total. The slow (aka "delayed") ion current is the potassium current, and its strength is proportional to the potassium electrochemical gradient and PK. It is now established that this current is eliminated by blockade of potassium channels with tetraethylammonium (TEA) applied externally and cesium ions (Cs+) applied internally.
The fast incoming INa and the slower outgoing Ik generally begin simultaneously, but INa develops faster and reaches its “ceiling”. Note that if E (MP) returns to the value characteristic of rest, then the sodium current disappears approximately 10 times faster than the potassium current. In other words, in bulk, membrane sodium channels are both activated (by depolarization) and deactivated (by repolarization) faster than potassium channels. Note that the activation of channels is determined by the opening of their activation gates, and deactivation by the closing of the same gates (Fig. 3.5).
Returning to the picture of the development of INa and IK during prolonged depolarization (Fig. ) under clamp conditions, let us note that INa disappears after 5 ms, despite the depolarized state of the membrane (IK remains intact). This phenomenon is called sodium channel inactivation. It is associated with the closing of special inactivation gates in sodium channels. The activation diagram of the activation gates of sodium channels (m-gates), as well as their inactivation gates (h-gates) at different MPs is shown in Fig. . Inactivation of sodium channels develops due to depolarization, i.e., apparently for the same reason as activation, but later, which makes INa possible (and therefore AP in vivo). Inactivation of PNa is a very important mechanism that contributes to the termination of the AP peak, which underlies inexcitability - refractoriness after excitation (refractory phase), cathodic depression and parabiotic state of the nerve (muscle) cell associated with depolarization. Note that inactivation of Na channels occurs after membrane repolarization. Inactivation of potassium channels in this object develops very slowly.
Fig.3.5. Scheme of the gate operation of voltage-gated sodium channels in the nerve membrane.
a - dependence of the percentage of open inactivation (h) and activation (τ) gates on the membrane potential, b - schematic representation of the positions of τ and h - gates at rest (1), development of the AP peak (2) and in the refractory phase (3).
Now let us turn to the characteristics of the natural ionic currents underlying the AP peak. These ionic currents are generally similar to those obtained during critical depolarization in the clamp technique, but they develop and cease much faster. The fact is that the development of AP is a strong change in MP, which affects transmembrane ion currents. At the same time, these ionic currents affect the MF.
In the process of development of PD, many factors operate, connected by direct and feedback connections.
For example, in the ascending phase of PD there is a system of factors with positive feedback:
Here, depolarization increases PNa, and the latter generates INa, which enhances depolarization (as long as INa is greater than Ik+I leakage). Due to these relations, INa in the AP and the ascending phase of the AP itself develops quickly, with acceleration, and the amplitude of the AP always reaches a certain maximum, more or less close to ENa (depending on the degree of compensation of the sodium current by K+ and leakage currents). During the decline of AP during repolarization, the same system of factors operates for INa, but with opposite signs of effects. And for the potassium current there is a system of factors with negative feedback:
As a result, the decline in Ik is somewhat delayed.
If we carefully take into account all these factors and their interaction, then it is possible to calculate the shape of a normal AP from the current values obtained when fixing the membrane potential at different levels, which is what A. Huxley and A. Hodgkin did. The coincidence of the calculated PD parameters and the PD parameters recorded in the experiment was shown.
The ion consumption per peak of one conducting AP is very small even in the squid giant axon. For example, the consumption of intra-axonal K is approximately equal to 1 millionth of the internal potassium reserve. Thus, the AP peak is a very economical signal that practically does not disturb the ion gradients on the membrane, the energy of which it is powered by. Ion gradients on the membrane are a “spring”, the energy of which can be enough for 5 • 105 PD without recharging. But for long-term operation of the fiber, ionic gradients need to be restored, which ensures the operation of the sodium-potassium pump of the membrane.
A few words about the mechanism of subthreshold local response (LR) in a nerve fiber. This response has basically the same mechanism as PD. Its ascending phase is determined by the incoming Na+ current, and its descending phase by the outgoing potassium current. But the amplitude of the LO (and its sodium current Na) is proportional to the strength of the subthreshold stimulation, and not standard, like that of the AP, i.e., it does not obey the “all or nothing” rule. LO does not cause absolute refractoriness, although it exhibits relative refractoriness due to inactivation of part of the sodium channels.
It is necessary to dwell on some features of action potentials in the cell bodies of mollusk neurons.
The bodies of giant neurons of gastropods are large structures that do not have synapses. These neurons have synapses on the so-called central process.
The large size and homogeneity of the surface make it possible to study the bodies of these neurons using the most accurate modern electrophysiological methods. Thus, with the help of two or more microelectrodes introduced into these cells, electrical stimulation of the cell is performed, its MPP and AP are recorded, Rm, Cτ are examined; The membrane potential is recorded and against this background the transmembrane currents underlying the excitation are studied.
In the laboratory of P. G. Kostyuk, a method of intracellular dialysis of a single giant nerve cell was developed. It consists of the following: a mechanically isolated giant nerve cell is placed in an appropriate saline solution in a chamber with flat walls, at the bottom of which there is a conical pore; its wide part (facing this vessel) can let the cell through, but its narrow part cannot. The pore walls are covered with a sticky mass. Under the chamber containing the cell there is another similar chamber (with the same saline solution), which communicates with the first only through the mentioned conical pore. The cell is brought to the pore and “sucked” into it, lowering the hydrostatic pressure in the lower vessel. In this case, parts of the cell that enter the pore adhere to its walls. The part of the cell protruding into the lower chamber is destroyed by passing a calcium-free solution through the lower chamber. After this, the hydrostatic pressure in the lower chamber and the environment in it are returned to normal. The result is a preparation of a somatic membrane, washed from the outside by the liquid of the upper chamber, and from the inside by the liquid of the lower chamber. Intracellular structures are washed away. Experiments have shown that using this somatic membrane preparation with electrical stimulation, full-fledged PD can be obtained. It was possible to clamp the potential of such a membrane, study transmembrane currents during its activation, and even obtain characteristics of the currents of individual membrane channels by reducing the area of the preparation.
The MPP of the soma is small (-40--50 mV) and has a mechanism generally similar to that known for axons. The PD is overshooted and reaches 100 or more millivolts (mV). It is similar in shape to an axon. But the ionic mechanisms of the AP membrane of the soma of the mollusk neuron have significant differences from those known for nerve fibers. The main difference is that the AP here is generated not only due to the incoming sodium current (as in the axon), but also due to the incoming calcium current. The somatic membrane contains special calcium channels, similar to sodium ones, but independent of them, which are opened by depolarization. The Ca2+ current through them occurs along an electrochemical gradient. These channels are not blocked by tetrodotoxin (TTX), but are blocked by cobalt, verapamil and the drug D-600. The calcium current develops more slowly than the sodium current and is inactivated much more slowly (many seconds!).
In addition, special “fast” voltage-gated potassium channels (which activate and inactivate very quickly) were found in the somatic membrane of the mollusk neuron. These channels are almost not involved in the formation of MPP and AP, because they are already inactivated at MP = 70 mV, i.e., at MPP. But these channels take part in the regulation of MP against the background of trace hyperpolarization, thereby determining to some extent the frequency of the rhythmic discharge in response to a particular influence.
Potassium channels involved in AP, i.e. channels of “delayed” outgoing current, also have a peculiarity here - they are inactivated faster than in the axon. Due to this, in somatic APs that follow rhythmically, the descending phases are progressively lengthened, but the amplitude is maintained, because the weakening of the potassium shunt compensates for the weakening of sodium currents from sodium inactivation. The characteristics of ion channels in the somatic membrane are likely to be important not only for electrogenesis. One might think that they are also essential for ensuring intensive metabolic reactions occurring in the soma, sensitive to [Ca2+], [K+] and [Na+] inside the cell. However, these characteristics of perikaryons are not the same in different animals. In the cell bodies of neurons in the spinal ganglia of rats, studied using the same method, no calcium channels involved in the generation of action potential were found; instead, special “slow” sodium channels were found there.
A few words should be said about the PD of nodes of Ranvier in nerve fibers of vertebrate animals. The action potentials of nodes of Ranvier have generally the same mechanism as that of the squid giant axon. However, the sodium current density of the AP is much higher here. The descending phase of AP is determined in interception not only by the outgoing potassium current, but to a large extent by a nonspecific leakage current. The role of this current is especially great in birds and mammals. According to recent data, myelinated mammalian nerve fibers have very few or even no voltage-gated potassium channels in the membrane of the nodes. But these fibers have such channels in the interstitial (internodal) membrane. Apparently, in all vertebrates, the nonspecific leakage current that determines the descending phase of AP in the interceptions of myelinated fibers also flows partly through the notches of the myelin sheath. In the genesis of the trace negative potential of a non-isolated interception of Ranvier, the accumulation of K+ ions in the interception gap plays a very important, if not the main, role.
In conclusion, let us dwell on the so-called noise of ion channels. Each of the ion channels of the membrane, which have certain gate mechanisms, even with a constant MP and chemical composition of the medium, is not always in one position; it opens and closes. These transitions from a closed state to an open state and back are carried out according to the law of chance and almost instantly, which determines the rectangular shape of a single current impulse in the channel. The open state time is called the “channel lifetime”. The chaotic opening and closing of channels causing ion movement creates electrical noise. For potential-dependent channels, the corresponding electric field is a factor that sharply increases the probability of the channels being open, which creates the effect of increasing ionic permeability. To study the dynamics of individual channels of a certain type, the weak electrical noise created by these channels in some small area of the membrane is studied under conditions of an MP clamp and chemical blockade of other channels. For example, in the membrane of the giant squid axon, the noise of sodium and potassium channels was studied separately.
Analysis of these noises made it possible to calculate the density of channels in the membrane and the conductivity of a single channel.
In the squid giant axon, the density of sodium channels turned out to be 300/1 μm2, and the channel conductance was 4 pmO. At the node of Ranvier in amphibians, the density of sodium channels is 2000/1 μm2, and the average channel conductance is 8 pmO.